2062
Late gadolinium enhancement imaging at low field using through-time spiral GRAPPA1Case Western Reserve University, Cleveland, OH, United States, 2National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3University of Michigan, Ann Arbor, MI, United States
Synopsis
A free-breathing late gadolinium enhancement scan may be beneficial for patients who have difficulty with breath-holds. However, the fast data acquisition needed may be difficult to achieve at low field strengths. Here, an undersampled spiral (R=3) acquisition with a through-time spiral GRAPPA reconstruction is used to achieve high spatial (1.25 x 1.25 mm2) and temporal (139 ms acquisition window) resolution for LGE imaging at 0.55T. The resulting images have qualitatively good contrast-to-noise between lesions and healthy myocardium, with acceptable SNR and without motion blurring.
Introduction
Low field MRI systems equipped with contemporary hardware and imaging methods can offer opportunities for cardiac imaging due to low cost, reduced SAR, reduced susceptibility and short T1/long T2* of tissue1. Spiral trajectories are especially amenable for low field imaging because the prolonged T2* enables long SNR-efficient readouts, and improved field homogeneity can reduce spiral image artifacts.Late gadolinium enhancement (LGE) is an important tool to assess myocardial infarctions and other tissue lesions2. Free breathing LGE offers benefits for patients unable to hold their breath2, but depends on high acceleration rates that may be challenging at lower fields due to coil geometry and low SNR. This study aimed to show the feasibility of an under-sampled spiral acquisition to achieve a high spatial and temporal resolution for LGE imaging at 0.55T.
Methods
Imaging was performed on an MRI system modified to operate at 0.55T (prototype MAGNETOM Aera, Siemens, Erlangen, Germany)3. The proposed sequence was tested in healthy volunteers, patients with known myocardial infarctions, and swine models of myocardial infarction. Human imaging was approved by local Institutional Review Board, and swine imaging was performed with local ethics approval.Data were acquired using a balanced steady-state free precession (bSSFP) sequence at end diastole following a non-selective adiabatic inversion pulse. Eight images were acquired with one recovery heartbeat between each, in a total breath-hold of 16 heartbeats. The final image was reconstructed from an average of the last 6 images, excluding the first 2 images to reach a steady T1 recovery signal. Imaging was performed 10 minutes following gadolinium administration (0.15 mL/kg gadobutrol).
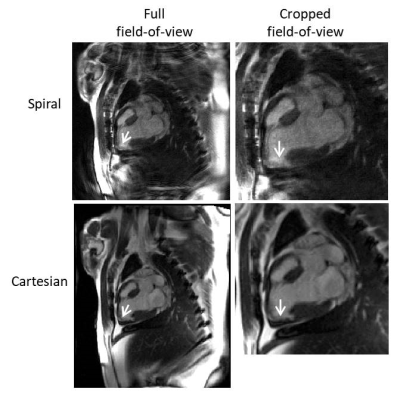
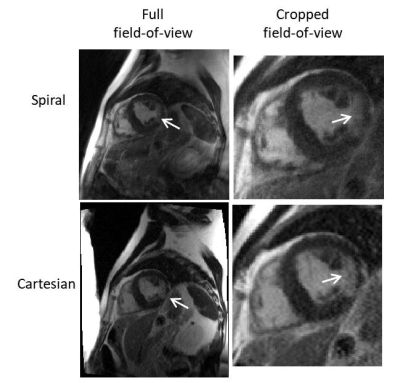
We used a 48 arm, variable density spiral-out trajectory deign4 with zeroth and first moment gradient nulling. Single-shot images were acquired with an under-sampling rate of 3 (16/48 arms) and reconstructed using through-time spiral GRAPPA5. Sixty fully-sampled, ungated calibration frames were acquired before or after the accelerated data (acquisition time ~ 60 seconds). An inversion pulse was played out every five frames to induce contrast variation in the calibration data. The scan parameters were: TR/TE = 8.66/1.04 ms, FOV = 320 x 320 mm2, matrix size = 256 x 256, in-plane resolution = 1.25 x 1.25 mm2, slice thickness = 8 mm, flip angle = 70°, dwell time = 2.5 μs, typical inversion time = 300 ms, temporal acquisition window = 139 ms.
Comparison images were acquired using a single-shot Cartesian phase sensitive inversion recovery bSSFP sequence2 with the following parameters: TR/TE = 3.4/1.35 ms, FOV = 380 x 323 mm2, in-plane resolution = 2.97 x 1.48 mm2, slice thickness = 8 mm, flip angle = 120°, temporal acquisition window = 320 ms. As with the spiral images, these data were acquired during a breath-hold.
Results
Figures 1 and 2 show example images from a swine model and from a patient with a known infarction, respectively. The images show qualitatively good contrast between regions of late enhancement and the healthy myocardium. The myocardium nulling is reduced with the spiral LGE sequence compared to conventional Cartesian LGE because the center of k-space is acquired with each spiral acquisition, meaning that the inversion time is spread over the 139 ms acquisition window. Using 6 averages, the SNR is acceptable at an in-plane resolution of 1.25 x 1.25 mm2. The short acquisition window permits visualization of the heart without blurring from myocardial motion.Discussion
This preliminary work demonstrates the use of an accelerated spiral acquisition and a through-time GRAPPA reconstruction to achieve high spatiotemporal resolution LGE images at 0.55T. Each average is acquired in a single heartbeat rather than in a segmented manner as a precursor to a free-breathing acquisition at low field. Toward this end, future work will include the addition of a motion correction algorithm to allow averaging of multiple, free-breathing averages. Quantitative SNR and contrast-to-noise evaluation of the images will also be performed.Conclusion
bSSFP LGE images can be acquired within a reasonable acquisition window (<200 ms) and at a diagnostic spatial resolution using an under-sampled spiral trajectory and a through-time spiral GRAPPA reconstruction at 0.55T.Acknowledgements
Funding was provided by the National Heart, Lung, and Blood Institute’s Division of Intramural Research. We would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHBLI and Siemens Healthcare.References
1. Simonetti OP, Ahmad R. Low-Field Cardiac Magnetic Resonance Imaging: A Compelling Case for Cardiac Magnetic Resonance’s Future. Circ. Cardiovasc. Imaging 2017;10:1–7 doi: 10.1161/CIRCIMAGING.117.005446.
2. Kellman P, Larson AC, Hsu L-Y, et al. Motion‐corrected free‐breathing delayed enhancement imaging of myocardial infarction. Magn. Reson. Med. 2005;53:194–200 doi: 10.1002/mrm.20333.
3. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology 2019;293:384–393.
4. Hargreaves B. Variable-Density Spiral Design Functions. http://mrsrl.stanford.edu/~brian/vdspiral/.
5. Seiberlich N, Lee G, Ehses P, Duerk JL, Gilkeson R, Griswold M. Improved temporal resolution in cardiac imaging using through-time spiral GRAPPA. Magn. Reson. Med. 2011;66:1682–1688 doi: 10.1002/mrm.22952.
Figures