2061
Rapid free-breathing 3D isotropic whole-heart diastolic and systolic myocardial T2mapping1Philips Japan, Tokyo, Japan, 2Department of Diagnostic Imaging and Nuclear Medicine, Tokyo Women’s Medical University, Tokyo, Japan, 3Department of Radiological Services,, Tokyo Women’s Medical University, Tokyo, Japan, 4Philips Healthcare, Hamburg, Germany, 5Philips Healthcare, Best, Netherlands
Synopsis
Current myocardial T2-mapping based on either segmented or single-shot readout obtains one 2D slice per breath hold. To overcome the limitations in spatial coverage and breath hold dependence, we report free-breathing 3D isotropic whole-heart T2 mapping using a T2-prepared segmented gradient echo sequence with interleaved scan, which enables quantitative mapping at multiple cardiac phases such as late diastole and systole. We demonstrate the feasibility of this approach with comparison to the conventional techniques in healthy volunteer examination.
Introduction
Quantitative myocardial T2-mapping is useful in diagnosis of heart diseases such as diffuse myocardial edema1-3. Current techniques that are widely used require reliable breath hold2,3. This often results in limited signal-to-noise ratio (SNR) and spatial coverage. A free-breathing 3D T2-mapping with whole heart coverage and isotropic spatial resolution, in the different heart-phases like CINE-imaging (diastolic and systolic phase, at least), would be clinically desired to accurately assess diffuse myocardial pathologies. In this study, we proposed a free-breathing whole-heart 3D T2-mapping, based on T2-preparation (T2prep)1 with relatively simple modification from routine CMR examination sequences, with isotropic spatial resolution in a clinically acceptable scan time.Methods
A schematic overview of the sequence for 3D isotropic myocardial T2-mapping is shown in Figure 1. T2-mapping was performed using a T2-prepared segmented RF-spoiled gradient echo (T1-turbo field-echo: T1TFE) sequence, similar to whole-heart coronary MRA or late gadolinium enhancement sequence at 3T. Recently, a unique T1ρ mapping approach using interleaved spin-lock prepared steady-state free precession pulse sequence has been proposed to achieve single breath-hold T1ρ-Mapping of the heart4, which employs different spin-lock pulses alternately before each TFE shots. We followed the same concept and replaced the spin-lock pulses by T2prep pulses. Furthermore, we applied 3D non-selective excitation pulses, resulting in improved image quality with much shorted TR/TE, hence higher TFE factor (echo train length) for the same acquisition window within each heartbeat, for balanced-TFE coronary MRA on 3.0T5,6. Besides, for robustness against B1 and B0 sensitivity across the entire the large-FOV, we applied a broadband offset independent trapezoid (OIT) pulse for two T2prep refocusing pulses, as already introduced for brachial plexus MR neurography7,8. Moreover, a combination of parallel imaging and compressed sensing technique (Compressed SENSE, C-SENSE) has recently been developed to accelerate the acquisition time without causing additional image artifacts9,10. we also applied C-SENSE for this T2-mapping sequence to further shorten the total scan time. For generating the T2map, four images with different T2-preparation times (TE = 0, 27, 53 and 80ms) were acquired with interleaved acquisition at the respective heartbeats. Each T2-weighted image was obtained with navigator respiratory triggering in both late diastole and systole timings separately. The repetition time (TFE shot interval) was set to 2 heartbeats resulting in a total scan time of less than 3minutes for each. To minimize the motion-induced misregistration, fast elastic image registration (FEIR)11 was employed for motion correction across the Prep-TE images.The study was approved by the local IRB, and written informed consent was obtained from all subjects. The scan parameters were optimized and compared with conventional T2-mapping sequence on a clinical 3.0T system (Ingenia, Philips Healthcare, Best, the Netherlands). Imaging parameters for 3D isotropic myocardial T2-mapping were; 3D non-selective T1TFE, Coronal acquisition, voxel size=2.0*2.0*2.0mm, FOV=340*340mm, C-SENSE reduction factor=9.0, TFE shot interval=2 heartbeats, flip angle=12°, TFE factor (average)=70, TR/TE=2.0/1.02ms, and total acquisition time=2 to 3minutes (4 to 6minutes for diastolic and systolic heart phases, depend on the body habitus).
Results
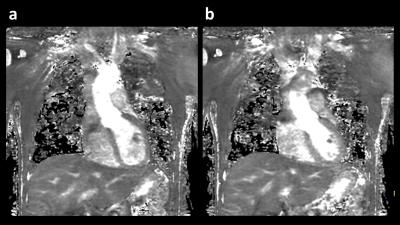
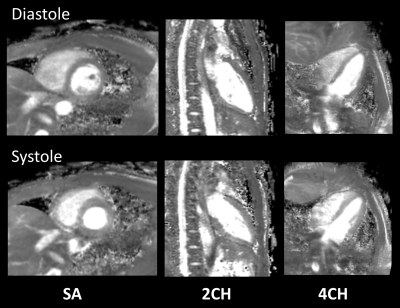
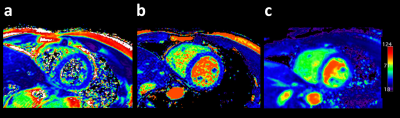
A total of 12 T2 maps were obtained in 6 healthy volunteers without known cardiovascular diseases, using the proposed sequence. Representative source images of 3D isotropic myocardial T2-mapping in diastole and systole are shown in Figure 2. These 2-mm isotropic images showed sufficient SNR and there were no obvious artifact in the heart. Figure 3 shows representative short-axis (SA), two-chamber (2CH), and four-chamber (4CH) MPR images in diastole and systole from the same 3D isotropic myocardial T2-mapping datasets. Figure 4 shows the comparison between 2D multi-echo gradient-spin-echo (mGraSE) [Fig 4a], single-shot T2prep-TFE [Fig 4b], and the proposed 3D isotropic myocardial T2-mapping method in SA at diastole [Fig 4c]. The measured T2 relaxation times in the ventricular septum from all the volunteers were: for mGraSE 52.5 ± 4.7ms, for 3D isotropic myocardial T2-mapping 53.9 ± 6.2ms. These values are comparable to the literature findings1,2.Conclusion
We demonstrated the feasibility of free-breathing whole-heart 3D T2-mapping with 2mm3 isotropic spatial resolution in the diastolic and systolic phases within a clinically acceptable scan time. It may be helpful for myocardial T2 assessment in patients with difficulties to hold their breath. More systematic investigations are needed to study its clinical robustness and quantification precision in comparison to the conventional techniques.Acknowledgements
No acknowledgement found.References
1. Giri S, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009 Dec 30;11:56.
2. Sprinkart AM, et al. Gradient Spin Echo (GraSE) imaging for fast myocardial T2 mapping. J Cardiovasc Magn Reson 2015;17:12.
3. Fernández-Jiménez R, et al. Fast T2 gradient-spin-echo (T2-GraSE) mapping for myocardial edema quantification: first in vivo validation in a porcine model of ischemia/reperfusion. J Cardiovasc Magn Reson 2015;17:92.
4. van Oorschot JW, et al. Single Breath-Hold T1ρ-Mapping of the Heart for Endogenous Assessment of Myocardial Fibrosis. Invest Radiol. 2016 Aug;51(8):505-12.
5. Kodaira K, et al. Acceleration of whole-heart coronary MR angiography using 3D non-selective bSSFP with Compressed SENSE. Proc. ISMRM:2019.2063.
6. Shiina I, et al. Whole heart coronary MRA using non-selective balanced SSFP sequence at 3.0T: comparison of image quality. Proc. ISMRM:2019.2073.
7. Yoneyama M, et al. Motion-sensitized driven-inversion (MSDI) for improvement of diffusion-prepared MR neurography (SHINKEI) in the brachial plexus. Proc Intl Soc Mag Reson Med. 2017;25:0854.
8. Yoneyama M, et al. Quantitative MR Neurography with robust fat suppression. Proc Intl Soc Mag Reson Med. 2018;26:5400.
9. Geerts-Ossevoort L, et al. Compressed SENSE Speed done right. Every time. The Netherlands: Philips Healthcare; 2018 Jan. Report No: 4522 991 31821. https://www.philips.de/content/dam/b2bhc/de/resourcecatalog/landingpages/ingeniaelition/White_Paper_Compressed_SENSE-opt.pdf
10. Sartoretti E, et al. Reduction of procedure times in routine clinical practice with Compressed SENSE magnetic resonance imaging technique. PLoS One. 2019 Apr 12;14(4):e0214887. doi: 10.1371/journal.pone.0214887. eCollection 2019.
11. Zhang S, et al. Cardiac magnetic resonance T1 and extracellular volume mapping with motion correction and co-registration based on fast elastic image registration. MAGMA. 2018 Feb;31(1):115-129.
Figures
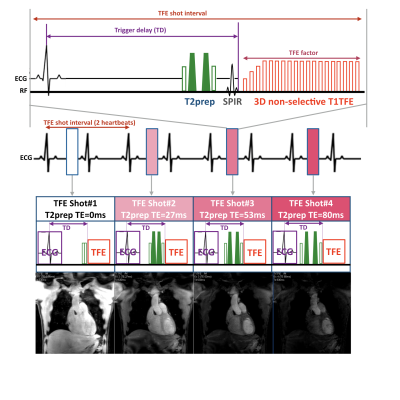
Figure 1. Scheme of the sequence for 3D isotropic myocardial T2-mapping.
T2-mapping was performed using a T2-prepared segmented gradient echo (turbo field-echo: TFE) sequence. 3D non-selective excitation pulses were also applied to shorten the TR/TE which leads to increase the TFE factor per one heartbeat. The numbers indicate the 4 images with different T2-preparation times (TE = 0, 27, 53 and 80ms). Each T2-weighted image was obtained with a navigator respiratory triggering and interleaved scanning, at both late diastole and systole timings separately.