2059
Improved Image Quality for 3D LGE Scar Imaging in Patients with Fast and Variable Heart Rate: a Simulation Study.1Cardiovascular Magnetic Resonance Unit, Royal Brompton and Harefield NHS Trust, London, United Kingdom, 2National Heart and Lung Institute, Imperial College London, London, United Kingdom
Synopsis
3D LGE is used to assess scar in patients with atrial fibrillation. However, the fast and variable heart rate in these patients results in poor image quality. An existing dynamic-TI method varies inversion time on a beat-by-beat basis (according to the previous cardiac cycle length) to improve myocardial nulling, but blood signal variations are incompletely corrected and cause ghosting. We have developed an improved technique which bases the beat-by-beat TI on the history of RR intervals (rather than the previous one) and reduces blood signal variations while maintaining myocardial nulling. Simulations with patient RR interval distributions show significantly improved results.
Introduction
3D LGE is a promising tool for assessing atrial scar distribution and burden in patients with atrial fibrillation (AF). However, single R-wave gating (needed to reduce study duration) coupled with the fast and variable heart rate in this patient population results in reduced image quality with poor myocardial nulling and ghosting.Techniques have been developed to improve image quality including saturation-inversion recovery [1], dynamic flip angle [2] and dynamic inversion time (dynamicTI) [3]. In the latter approach, the inversion time is varied on a beat-by-beat basis to improve myocardial nulling, according to the length of the previous cardiac cycle. However, variations in the blood pool signal are incompletely corrected (due to its different T1) and ghosting is still an issue.
In this work, we develop a new dynamicTI technique which (i) bases the beat-by-beat TI calculation on the history of RR intervals (rather than just the previous one) and (ii) reduces the blood pool signal variations through the acquisition (rather than the myocardial signal variations) while maintaining the myocardial signal close to zero.
Methods
Our proposed method uses the RR interval history up to any given cardiac cycle to determine the TI for that cardiac cycle to achieve consistent blood signal throughout the acquisition. To do this we use Bloch simulations of the 3D inversion-prepared segmented gradient echo sequence (TR 3.5ms, views per cardiac cycle 37, flip angle 20, centric ky inside centric kz) to model the longitudinal magnetisation (Mz) throughout the acquisition.We developed a 3D numerical phantom of a left ventricular short axis volume (blood T1 = 420ms, scar T1 = 360ms, myocardium T1 = 490ms) and performed MATLAB simulations using (i) fixedTI (based on the average RR interval through the acquisition), (ii) original dynamicTI [3] and (iii) new dynamicTI using a patient-specific target Mz for blood. The target blood Mz was based on the mean RR interval over the first 30 cardiac cycles and is the blood Mz at the myocardium null time at that mean RR interval. Simulations were performed using RR interval data from 43 patients with persistent AF who attended for 3D LGE prior to RF ablation and from 14 patients post RF ablation. For each dataset, the standard deviation (SD) of the RR intervals was used as a measure of RR interval variability. For each simulation, mean myocardial (myo) signal, mean ghosting signal, scar-blood contrast-to-ghosting ratio (CGR) and scar-myo CGR were calculated. ANOVA and subsequent paired t-testing (with Bonferroni correction) were used to assess statistical differences between the three methods.
Results
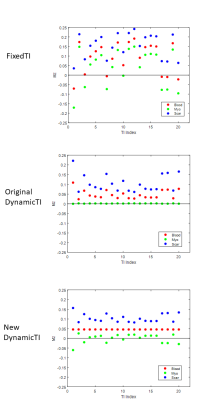
Figure 1 shows blood, myo and scar Mz for the most central k-space line in each of 20 cardiac cycles in an example simulation. While the original dynamicTI method aims to reduce the Mz variations of myo, Fig. 1 shows that the new method reduces the Mz variations of the blood pool with a view to reducing blood pool ghosting.Simulations using all three methods are shown in an example post-ablation case in Fig. 2, together with the associated RR interval histogram and the ghosting signal histograms. Both dynamicTI techniques improve myocardial nulling and reduce ghosting compared to the fixedTI technique, with the ghosting level for the new dynamicTI technique being less than that of the original dynamicTI technique. This is more apparent in the pre-ablation example in Fig. 3 where there were multiple missed cardiac triggers.
The results of the pre-ablation and post-ablation simulations are shown in Table 1. Compared to fixedTI, both dynamic TI methods improve scar-blood and scar-myo CGR and improve myocardial nulling. Comparing the new and original dynamicTI methods, pre-ablation ghosting signal is reduced (0.22 +/- 0.06 vs 0.18 +/- 0.03, p<0.0001) and both scar-blood CGR and scar-myo CGR are improved (22.5 +/- 8.2 vs 26.2 +/- 6.8, p<0.001 and 40.2 +/- 14.9 vs 45.7 +/- 12.8, p<0.001 respectively). Myo signal nulling is maintained (0.17 +/- 0.04 vs 0.18 +/- 0.03, p=ns). Similar trends are shown in the post-ablation simulations although these are less marked as the RR interval variability is less than in the pre-ablation simulations (RR interval variability: 158ms +/- 85ms vs 287ms +/- 80ms.).
Discussion
Our new dynamic TI method, which targets a patient-specific constant blood pool signal through the acquisition, significantly reduces ghosting and improves both scar-blood and scar-myo CGR compared to the original method which targets a constant myocardial signal. In future work the proposed approach will be integrated into an imaging sequence, for phantom and in vivo validation.A disadvantage of our new technique (as with the original dynamicTI technique) is that it targets a single T1 , allowing signal variations from different T1 materials to persist. However, by targeting the T1 of blood (which is responsible for most of the ghosting signal) rather than myocardium, the image improvement (in terms of reduced ghosting and improved contrast) is greater. Compared to methods that introduce partial [2] or full [1] saturation pulses, SNR is not inherently reduced by our new method.
Acknowledgements
This work was supported by the British Heart Foundation (BHF).References
1. Weingärtner et al. MRM, 71(3), 1024–1034. (2014)
2. Hu et al. JMRI, 49(3), 688–699. (2019).
3. Keegan et al. MRM, 73(2), 646–654. (2015).
Figures