2058
Fast single-breathhold 2D multi-slice myocardial T1 mapping (FAST1) at 3T for time-efficient full left ventricular coverage
Li Huang1, Radhouene Neji1,2, Muhummad Sohaib Nazir1, Amedeo Chiribiri1, Reza Razavi1, and Sébastien Roujol1
1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Modified Look-Locker inversion recovery (MOLLI) as a commonly used myocardial T1 mapping approach shows high precision and reproducibility. Its limited capability in multi-slice acquisition per breathhold prolongs examination time in cases of desired full left ventricular coverage. The previously proposed fast single-breathhold 2D multi-slice myocardial T1 mapping (FAST1) technique can achieve time-efficient full left ventricular coverage at 1.5T. In this work, this capability of FAST1 at 3T is optimized and characterized. Compared to MOLLI, FAST1 can yield 4-fold increase of spatial coverage, limited penalty of T1 spatial variability, no significant difference of T1 repeatability and linearly correlated T1 values.
Introduction
Myocardial T1 mapping shows promise in assessing cardiomyopathies1. The modified Look-Locker inversion recovery (MOLLI)2-3 technique is commonly used due to high precision and reproducibility4-6. In MOLLI and its variations such as ShMOLLI7, generation of a single T1 map requires 7-13 image acquisitions per breathhold within 9-17 heartbeats (HBs), thus limiting their applications for full left ventricular (LV) coverage. The previously proposed fast single-breathhold 2D multi-slice myocardial T1 mapping (FAST1)8 technique can achieve full LV coverage in three breathholds at 1.5T. In this work, we sought to optimize and characterize the capability of FAST1 for time-efficient full LV coverage at 3T.Methods
(1) Sequence: The FAST1 sequence was modified for imaging at 3T to enable the acquisition of four slices per breathhold (Fig. 1). In each 3-HB imaging block for a single slice, 3 ECG-triggered slice-selective inversion recovery (IR) prepared 2D bSSFP readouts are performed at different inversion times (TIs). For suppressing motion-induced partial volume effects between inversion and acquisition, the first TI is minimized and the inversion/imaging slice thickness ratio (RTHK) is optimized (4)8. Combined with slice interleaving, NR recovery HBs are inserted between the chronologically second and third imaging blocks to ensure recovery delay TRD between inversion pulses for adjacent slices, in order to overcome slice cross-talk8. TRD is optimized to be at least 7s (~5·typical myocardial T1 time 1400ms at 3T), allowing quasi full recovery of the longitudinal magnetization of myocardium in the worst-case scenario (defined as a slice being fully inverted by an adjacent slice inversion)8. Compared to FAST1 sequence at 1.5T8, the number of images per slice is increased from 2 to 3 for compensating precision loss due to longer myocardial T1 times at 3T. Thus the number of slices in FAST1 is reduced from 5 at 1.5T to 4 at 3T to maintain clinically acceptable breathhold duration (~13s).(2) Reconstruction: T1 maps are reconstructed using a dictionary matching approach8-9 succeeding a phase-sensitive inversion recovery approach10. The signal dictionary is generated using a one-parameter model8-9 defined as S(TI)=1-(1+δ)e-TI/T1 with δ as the inversion factor of the inversion pulse8-9,11. δ=0.9133 was computed using Bloch equations simulation of the inversion pulse in predefined typical T1/T2/B0/B1 regimes across myocardium at 3T (600-1800ms/50ms/±300Hz/60-100%, estimated according to literature12-13). Heart rate (HR) correction for T1 maps was applied using a previously-proposed model based on independently-obtained phantom data8-9.
(3) Simulation: Accuracy and precision of FAST1 using 2 images only (1.5T version8) and 3 images (as proposed here for 3T) were evaluated and compared to conventional 5-(3)-3 MOLLI using Bloch equations based Monte Carlo simulation (N=5000) with T1/T2 ranges of 500-1700ms/35-65ms and signal-to-noise ratio of 50.
(4) Experiments: FAST1 (3 images/slice) and conventional 5-(3)-3 MOLLI were performed on a 3T scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany) in phantom14 using a simulated HR of 60bpm and 7 healthy volunteers (5 male, 30±2yrs). Both sequences used the same 2D bSSFP parameters: TR/TE/α=2.70ms/1.12ms/35°, FOV=360×306mm2, pixel size=1.4×2.1mm2, slice thickness=8mm, GRAPPA factor=2, partial Fourier factor=7/8, bandwidth=1085Hz/px, first TI=100ms. For the healthy volunteer study, slices were prescribed in the short-axis orientation, and FAST1/MOLLI were performed 3/12 times each at different slice positions to generate 12 contiguous slices for full LV coverage within 3/12 breathholds. 5/2 scan repetitions were performed in phantom/healthy volunteers, respectively.
(5) Analyses: T1 times/spatial variability/repeatability of FAST1 and MOLLI were measured in each vial (phantom) and each myocardial segment using the 16-segment model15 on 3 representative slices (basal/mid-ventricular/apical) in both FAST1 and MOLLI datasets (healthy volunteers). 0/4 of 21 slices in FAST1/MOLLI datasets showed substantial respiratory motion due to breathholding failure. To prevent any bias, the 4 motion-corrupted slices in MOLLI were discarded from both MOLLI and FAST1 datasets for subsequent statistical analyses.
Results
(1) Simulation study (Fig. 2): 2-image FAST1, 3-image FAST1 and MOLLI led to mean errors of 5% vs. 4% vs. 4%. With respect to MOLLI, 2- and 3-image FAST1 showed reduced precision by factors of 1.5 and 1.4 in the entire investigated T1/T2 ranges, respectively.(2) Phantom study (Fig. 3): FAST1 and MOLLI yielded accuracy of -50±51ms vs. -43±49ms, spatial variability of 5±2ms vs. 4±2ms, repeatability of 2±1ms vs. 1±0ms, respectively.
(3) Healthy volunteer study (Fig. 4 and Fig. 5): The healthy volunteers had the HRs of 59±8bpm. Compared to MOLLI, FAST1 yielded decreased native T1 times (1172±42ms vs. 1221±41ms, p<0.0001), higher spatial variability by a factor of ~1.2 (57±18ms vs. 50±11ms, p<0.001) and no significantly different repeatability (14±10ms vs. 14±12ms, p=0.99). A Pearson correlation coefficient of 0.79 was found between native T1 times using both techniques.
Discussion
No motion correction was performed in FAST1 and integration of an image registration algorithm16-17 will be investigated in the future to further improve the quality of FAST1 maps. FAST1 does not allow for estimation of blood T1 due to the use of a slice-selective inversion pulse. The validation in a large patient cohort, particularly where regional T1 variations are expected, will be the focus of future work.Conclusion
FAST1 yields a 4-fold increase of spatial coverage, limited penalty of T1 spatial variability, no significant difference of T1 repeatability and linearly correlated T1 values in comparison with MOLLI at 3T. FAST1 enables full LV coverage in 3 breathholds.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/R010935/1), the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London, and Siemens Healthcare. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
- Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15(1):92.
- Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141-146.
- Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003;5(2):353-359.
- Roujol S, Weingärtner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272(3):683-689.
- Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16(1):2.
- Weingärtner S, Meßner NM, Budjan J, Loßnitzer D, Mattler U, Papavassiliu T, Zöllner FG, Schad LR. Myocardial T 1-mapping at 3T using saturation-recovery: reference values, precision and comparison with MOLLI. J Cardiovasc Magn Reson. 2017;18(1):84.
- Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12(1):69.
- Huang L, Neji R, Nazir MS, Whitaker J, Duong P, Reid F, Bosio F, Chiribiri A, Razavi R, Roujol S. FASt single-breathhold 2D multi-slice myocardial T1 mapping (FAST1) at 1.5T for full left ventricular coverage in three breathholds. J Magn Reson Imag. 2019;epub.
- Huang L, Neji R, Nazir MS, Whitaker J, Reid F, Bosio F, Chiribiri A, Razavi R, Roujol S. Fast myocardial T1 mapping using shortened inversion recovery based schemes. J Magn Reson Imag. 2019;50(2):641-654.
- Xue H, Greiser A, Zuehlsdorff S, Jolly MP, Guehring J, Arai AE, Kellman P. Phase-sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magn Reson Med. 2013;69(5):1408-1420.
- Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med. 2014;71(4):1428-1434.
- Kellman P, Herzka DA, Arai AE, Hansen MS. Influence of Off-resonance in myocardial T1-mapping using SSFP based MOLLI method. J Cardiovasc Magn Reson. 2013;15:63.
- Sung K, Nayak KS. Measurement and characterization of RF nonuniformity over the heart at 3T using body coil transmission. J Magn Reson Imag. 2008;27:643-648.
- Captur G, Gatehouse P, Keenan KE, Heslinga FG, Bruehl R, Prothmann M, Graves MJ, Eames RJ, Torlasco C, Benedetti G, Donovan J, Ittermann B, Boubertakh R, Bathgate A, Royet C, Pang W, Nezafat R, Salerno M, Kellman P, Moon JC. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance-the T1 mapping and ECV standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18:58.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539-542.
- Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67:1644-1655.
- Roujol S, Foppa M, Weingärtner S, Manning WJ, Nezafat R. Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): Application to T1 mapping. Magn Reson Med. 2015;73:1469-1482.
Figures
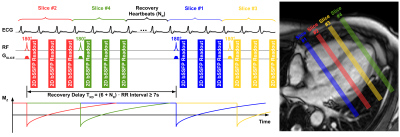
Fig. 1. FAST1 sequence diagram at 3T. Four
slices are acquired in one breathhold. Each slice is acquired over three
consecutive HBs following application of a slice-selective inversion pulse. A
short first TI and thickened tagging (RTHK=inversion/excitation
slice thickness ratio) are used for suppressing motion-induced partial volume
effects between inversion and acquisition. Slice interleaving and insertion of
NR recovery HBs are applied for overcoming slice cross-talk.
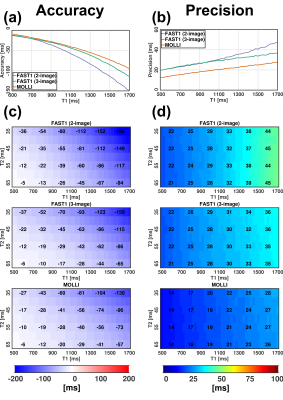
Fig. 2. Results of the simulation study. T1-dependent
accuracy (a) and precision (b) at T2=50ms, as well as T1-T2
dependent accuracy (c) and precision
(d) of 2-image FAST1, 3-image FAST1
and MOLLI in the presence of added noise are shown, respectively. Accuracy is calculated
as T1 mean difference to actual T1 while precision is calculated as T1 standard
deviation (SD) over N=5000 simulation repetitions.
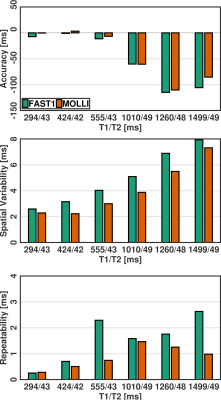
Fig.
3. Results
of the phantom study. Region of interest (ROI) analysis was performed in 6
vials representing typical native and post-contrast T1 mapping (reference T1/T2
values as the horizontal axes) in phantom. Accuracy (average of T1 mean
difference in ROI to reference T1 over repetitive scans), spatial variability
(average of T1 SD in ROI over repetitive scans) and repeatability (SD of T1
mean in ROI over repetitive scans) are shown.
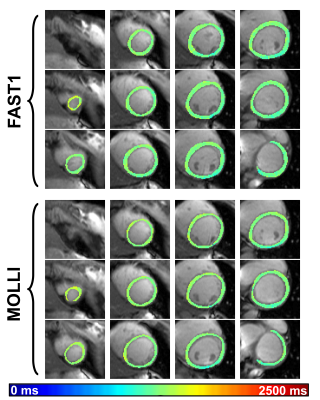
Fig.
4. Representative
example native T1 maps of a healthy volunteer using FAST1 and MOLLI. Note that each
row of FAST1 T1 maps and each MOLLI T1 map represent a sequence run within one
breathhold, respectively.
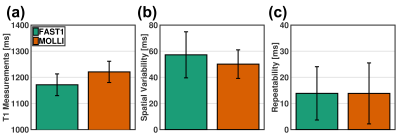
Fig.
5. Data
analyses in healthy volunteer study in terms of native T1 times (a), spatial variability (b) and repeatability (c). Native T1 times is averaged T1
values in segment over repetitive scans, spatial variability is computed by
averaging T1 SD in segment over repetitive scans, and repeatability is
calculated as SD of T1 mean in segment over repetitive scans.