2057
Impact of calibration-based coil combination on myocardial fibrosis assessment with a 3D Delayed Myocardial Enhancement sequence prototype1ASL MR, GE Healthcare, Barcelona, Spain, 2Hospital Clínic de Barcelona, Barcelona, Spain, 3GE Healthcare, Munich, Germany
Synopsis
We present the results of a comparative study of the impact of clinically available MR coil combination methods on the diagnostic value of 3D Delayed Myocardial Enhancement data, with a focus on arrhythmogenic tissue analysis.
INTRODUCTION:
The use of Delayed Myocardial Enhancement sequences has become a well-established technique for the determination of tissue viability in ischemic cardiomyopathies1. Imaging is based on the use of T1-prepared MR pulse sequences to determine the distribution of a Gadolinium-based contrast agent in the myocardium. In order to attain the optimal contrast between viable and pathologic tissue, the acquisition is performed after allowing the contrast agent to redistribute for approximately 15 minutes.Due to the diagnostic relevance of subtle uptake patterns, achieving a good intensity contrast in the myocardium is critical for the success of these exams. This is limited by the accuracy of the inversion time parameter, which determines the suppression of healthy myocardium signal, and the overall signal-to-noise ratio of the image.
Advanced coil combination methods incorporating calibration information are known to provide improved noise performance, in comparison to conventional sum-of-squares methods. On the other hand, these methods are susceptible to anatomical misalignment with the calibration series.
In this study, we examine the impact of two different coil combination approaches on the diagnostic value of Delayed Myocardial Enhancement images acquired with a three-dimensional pulse sequence prototype. Particular attention is given to the outcome of automated clinical metrics of the arrhythmogenic properties of tissue, used to drive therapeutic decision-making.
METHODS:
This study was performed by retrospectively reconstructing 3D-DME data from 15 subjects (12M/3F, age 56±14, weight 78±13kg) referred for a clinically-indicated contrast-enhanced cardiac MRI examination at the Clinical Hospital of Barcelona.Acquisitions were performed on a General Electric Architect 3T scanner using a prototype pulse sequence with the following parameters: 3D SPGR readout; inversion-recovery T1 preparation with TI 283±29ms determined using a CINE scout scan; 40cm field-of-view R/L; matrix 320x320; ST 2.0-2.4mm; bandwidth 62.5kHz; TE 2.2ms; spatial and chemical saturation; ARC acceleration; respiratory navigator; ECG triggering with trigger time 616±138ms.
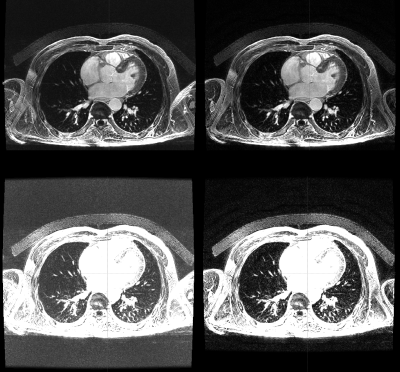
Cartesian 3D image reconstruction was performed offline using GE’s Orchestra libraries on the raw data of the Delayed Enhancement scan and those of a coil-matched calibration series, acquired during the same exam. Two coil channel combination approaches were tested: Standard sum-of-squares; and a calibration-based method akin to that used for ASSET acceleration.
The resulting datasets were examined by two board-certified cardiologists. Image quality was assessed in terms of signal and noise levels in the background, blood pool and myocardium. They were also compared in terms of artifacts and morphology of the ventricles and atria, as well as pathological uptake when present.
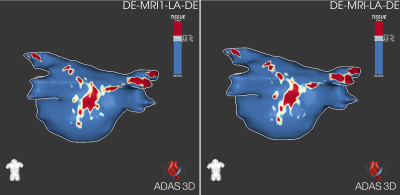
Further analysis was performed using the ADAS arrhythmic substrate identification software (Galgo Medical, SL). The total myocardial area, border zone and lesion core percentages obtained with both reconstructions, as well as the overall lesion distribution, were compared in all cases2.
RESULTS:
All cases were successfully exported and reconstructed with both coil combination methods. Qualitatively, the most obvious difference between the methods was a reduction of wrap-around artifacts (found in the arms region with our acquisition settings), sometimes replaced by a slight decrease of signal intensity in that region. Narrower intensity windows revealed improved noise performance in the calibration-based method.This was confirmed by the results of region of interest analysis: Background average decreased by a factor 0.3±0.3 with the calibration-based method, myocardium signal decreased by a 0.8±0.2 and blood signal remained within 1.0±0.0. Wrap-around artifacts decreased in all cases and motion artifacts in 64%. The reconstructions were found to be morphologically equivalent in all the cases for the left ventricle, right ventricle, right atrium and left atrium, respectively. Pathological uptake was identified in 2 cases, and both of them were qualified as diagnostically equivalent with both reconstruction methods.
The quantitative results provided by ADAS segmentation were found to differ by less than 5% between the reconstructions, despite being segmented independently: Total area 0.5±2.2cm2; Border zone 0.4±0.5cm2, 0.3%±0.4%; Core zone 0.0±0.5cm2, -0.1%±0.9%. The arrhythmic substrate pattern was qualitatively equivalent in all cases.
DISCUSSION:
The results obtained in this evaluation study suggest that, while both coil combination techniques are suitable for diagnostic 3D-DME imaging, using a calibration-based method offers reduced noise and artifacts without any noticeable drawbacks. A suitable calibration scan is certainly required, but can be readily acquired in a few seconds, when not already available from previous sequences (e.g. those using image-space acceleration or calibration-based homogeneity correction).No incidences were found of diagnostically relevant artifacts caused by anatomical mismatch between the calibration and DME series. While the limited number of cases included in this preliminary study does not allow to draw general conclusions, the absence of noticeable artifacts is a relevant finding, considering that only pre-existing calibration series were used, typically acquired before contrast injection.
Concerning the impact on the dedicated post-processing for arrhythmogenic tissue characterization, the differences between the reconstructions are likely to be dominated by the reproducibility of the segmentation3. These results indicate that advanced coil combination methods can be implemented without changing the analysis and therapy guidance protocols currently implemented by the electrophysiology team.
CONCLUSION:
The results of this comparative study indicate that the well-known benefits of calibration-based coil combination extend to three-dimensional DME imaging. No instances of artifacts caused by calibration misalignment were encountered. The use of different coil combination approaches didn’t affect the outcome of arrhythmogenic pathway analysis.Acknowledgements
No acknowledgement found.References
1. Vogel-Claussen, J. et al. Delayed Enhancement MR Imaging: Utility in Myocardial Assessment1. RadioGraphics (2006) doi:10.1148/rg.263055047.
2. Benito, E. M. et al. Left atrial fibrosis quantification by late gadolinium-enhanced magnetic resonance: a new method to standardize the thresholds for reproducibility. Europace 19, 1272–1279 (2017).
3. Margulescu, A. D. et al. Reproducibility and accuracy of late gadolinium enhancement cardiac magnetic resonance measurements for the detection of left atrial fibrosis in patients undergoing atrial fibrillation ablation procedures. Europace 21, 724–731 (2019).
Figures