2054
Fast T1rho mapping in mice using an optimized Bloch simulation based radial sampling pattern1Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany, 2Experimental Physics 5, University of Würzburg, Würzburg, Germany, 3Comprehensive Heart Failure Center (CHFC), University Hospital Würzburg, Würzburg, Germany
Synopsis
Myocardial T1ρ-mapping in small animal studies is still a challenging procedure. Commonly, T1ρ-mapping requires long measurement times or only provides insufficient image quality due to a low signal-to-noise-ratio. Using a novel approach based on an optimized radial sampling pattern and high flip angles, we were able to overcome both restrictions. By a special sorting of golden angles based on Bloch simulations the image quality of the method could be significantly increased, and a high quantification accuracy could be realized. Thus, the new approach is a reliable method for fast T1ρ-mapping in future studies on small animals.
Introduction
In the field of cardiac MRI, numerous studies have pointed to the alternative contrast mechanism of the spin-lattice relaxation time T1ρ under the spin-lock (SL) condition [1,2,3]. It has been shown that T1ρ provides a high endogenous contrast between healthy myocytes and collagenous scars in cardiac tissue [1]. For this, myocardial T1ρ-mapping is of great interest for improved imaging and diagnostics of cardiac diseases in animal models. Due to the high heart rate and the special concept of SL preparation, acquisition of T1ρ-maps in small animals is quite challenging. Hence, commonly used T1ρ-mapping sequences require long measurement times or only provide an insufficient image quality due to a low signal-to-noise-ratio.Therefore, it is crucial to establish new methods to overcome both restrictions generating accurate and high-resolution T1ρ-maps within very short scan times. In this work, we present a new highly accelerated and optimized approach for myocardial T1ρ-mapping that allows the acquisition of a T1ρ-map in less than two minutes.Methods
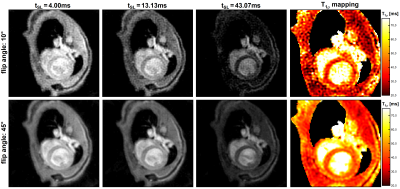
All measurements were performed on a 7.0T small animal imaging system (Bruker BioSpec 70/30, BioSpin MRI GmbH, Ettlingen, Germany). Acquisition of k-space was optimized for very fast myocardial imaging using a radial spoiled gradient echo readout (TE=1.9ms, TR=4.7ms). T1ρ preparation was performed by totally-balanced-spin-locking (TB-SL) [4]. A key point of the new concept was to increase the SNR by using high flip angles (α=45°) in the readout. Due to the resulting high signal changes during data acquisition, an optimized sampling scheme is required. For every T1ρ-weighted image, data sampling was segmented into 13 preparation experiments (with identical preparation times tSL), acquiring four radial spokes after the SL preparation (Fig. 1). In order to generate T1ρ-maps, a series of 8 T1ρ-weighted images with different tSL is acquired, leading to 13x8=104 consecutive preparation experiments in total. The acquisition window was positioned in end diastole using a suitable trigger delay (depending on tSL). Each preparation experiment was separated by a waiting time trec for magnetization recovery, which is dependent on the respiratory cycle rate resulting in a total measurement time of approximately 2min.For k-space sampling, three different golden angle sorting schemes were used and compared (Fig. 2). First a serial sampling, at which subsequent golden angles were increased in every subsequent TR. Second an echo number based sampling, at which subsequent golden angles were assigned to the acquisition number after the preparation module. And finally, our novel Bloch simulation based sampling, at which subsequent golden angles were assigned to the expected signal intensity of the corresponding acquisition window. For this, the signal intensity for every acquisition window is simulated using the known sequence timings and estimated T1 and T1ρ values of the probe under investigation. For all three sampling schemes, image reconstruction was performed using a KWIC filtered view sharing method [5]. Here, the k-space center for a desired T1ρ-weighting (with desired tSL) is exclusively selected from the first acquisition window after preparation. The k-space peripherals were also chosen from other acquisition windows as well as other T1ρ-weightings.The three sampling schemes were compared in phantom measurements consisting of four cylindrical sample tubes with different concentrations of BSA (Bovine Serum Albumin) analyzing the SNR and artifact susceptibility of the methods. Furthermore, myocardial T1ρ-mapping with our Bloch simulation based sampling scheme was performed on mice. The achievable image quality was compared with the echo based sampling using low flip angles (10°).
Results
Fig. 3 shows the results of the SNR and artifact analysis. Our optimized Bloch simulation sorting of the golden angles causes a significant increase in SNR by a factor of 3.09 compared to the serial sorting and by a factor of 1.34 in relation to the echo position sorting. Analysis of the T1ρ quantification accuracy revealed a maximum deviation of smaller than 1.7% in comparison to a cartesian fully sampled reference scan (Tab. 1) using our optimized Bloch simulation sampling. The results of the in vivo study show that the use of low flip angles does not provide sufficient SNR for myocardial T1ρ-mapping. In contrast, our new method based on high flip angles and Bloch sorting provides a significantly higher image quality. Compared to the cartesian reference scan, the measurement time was reduced by a factor of 1.8 to less than 2 minutes (depending on the respiratory cycle).Discussion
In the presented work, we introduced a novel fast myocardial T1ρ-mapping sequence using an optimized sampling scheme and high flip angles. The phantom and in vivo measurements showed that our new method provides a high SNR and excellent image quality. Using significantly undersampled radial data, a very short measurement time of less than 2 minutes could be achieved. In addition, the use of a radial data acquisition results in a higher robustness against motion artifacts. Due to the very short measurement time, our method enables further detailed T1ρ quantification and even T1ρ dispersion measurements in vivo during a practicable small animal study protocol. In future measurements, the quantification of T1ρ in the myocardium of mice will be studied in detail in specific heart diseases. An ongoing study is focused on myocardial ischemia associated with Fabry disease.Acknowledgements
This work was supported by the Federal Ministry for Education and Research of the Federal Republic of Germany (BMBF 01EO1504, MO6).References
[1] Witschey et al. Magn Reson Med. 2010 Nov;64(5):1453-60
[2] van Oorschot et al. J Magn Reson Imaging. 2015 May;41(5):1181-9
[3] Kamesh Iyer et al. J Cardiovasc Magn Reson. 2019 Jan 10;21(1):5
[4] Gram et al. ISMRM Annual Meeting 2019. Montreal. #1215
[5] Song et al. Magn Reson Med. 2000 Dec;44(6):825-32.
Figures