2045
3D Whole-heart Motion Compensated Grey-blood Late Gadolinium Enhancement Imaging1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Grey-blood phase-sensitive inversion-recovery (PSIR) late gadolinium enhancement (LGE) imaging has shown promising and robust results for the assessment of myocardium viability. Conventionally 2D grey-blood LGE images are acquired under several breath-holds in different image orientations to depict scar extension. However, these approaches achieve limited spatial resolution and coverage and can be challenging in un-collaborative patients. In this work, we have proposed a free-breathing 3D whole-heart LGE sequence with water/fat Dixon encoding and blood nulling which provides grey-blood PSIR images with isotropic resolution for scar visualization and complementary 3D fat images for pericardial and myocardial adipose tissue detection in ~6min.
Introduction
Late gadolinium enhancement (LGE) cardiovascular MR plays an important role in the detection and assessment of myocardial viability. Particularly, grey-blood phase-sensitive inversion-recovery (PSIR) LGE imaging has shown promising and robust results due to increased contrast-to-noise ratio (CNR) between scar, normal myocardium and blood, overcoming most of the limitations of bright-blood LGE such as endocardial scar detection1,2. Conventionally, 2D grey-blood LGE images are acquired over multiple breath holds in different image orientations to visualise and quantify scar extension. However, this approach is limited by image misalignment, anisotropic resolution and the need for multiple breath-holds. Furthermore, the presence of myocardial fat infiltration or epicardial fat may render the distinction between fat and LGE challenging both in ischemic and non-ischemic cardiomyopathy3. Here we propose a novel free-breathing 3D whole-heart LGE sequence with water/fat Dixon encoding and magnitude blood-nulling which provides a grey-blood PSIR volume, for scar visualization and complementary 3D fat images for fat detection. The proposed approach was evaluated in a T1 phantom and 6 patients with suspected cardiovascular disease and compared against standard 2D grey-blood LGE with blood-nulling.Methods
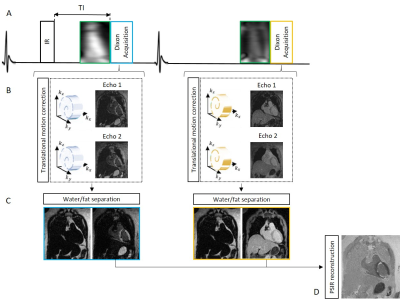
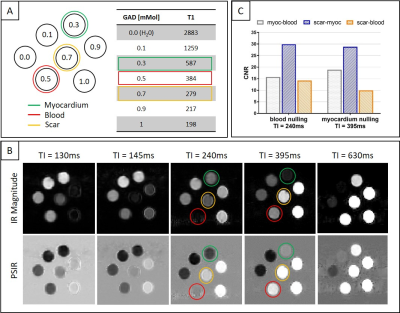
The framework of the proposed sequence is shown in Fig.1. Two interleaved 3D volumes are acquired with ECG-triggered two-point bipolar Dixon, spoiled gradient echo readout and 3x undersampled variable-density Cartesian spiral-like trajectory4,5. The two datasets are acquired with 1) Inversion Recovery (IR) preparation and 2) no preparation. Low-resolution 2D image navigators (iNAVs) are acquired prior to imaging to correct translational respiratory motion, enabling 100% scan efficiency6. Translational motion correction is performed for each echo, and each undersampled in- and out-of-phase volume is reconstructed with iterative-SENSE7. A water-fat separation algorithm is used to generate fat and water images for both datasets8 and a PSIR reconstruction is performed to obtain the grey-blood LGE volume.Acquisition: A T1 phantom composed of 7 vials (including T1s of post-contrast myocardium, blood and scar) filled with different gadolinium concentrations and six patients (4 male, 63±7yo) were scanned on a 1.5T scanner (Siemens Magnetom Aera). Acquisition parameters included: FA=25deg and 5deg for IR-prepared and non-prepared dataset, isotropic resolution of 2mm3, FOV=320x320x104-128mm3, 14 low flip angle echoes (FA=3deg) for iNAV acquisition, subject specific mid-diastolic trigger-delay and acquisition window of 102-122ms, TR/TE1/TE2=6.41/2.38/4.76ms, bandwidth=602Hz/pixel and total scan time=6±1min42sec. A 2D scout scan with imaging parameters matching the 3D LGE sequence was performed prior to each patient acquisition to select the optimal inversion time (TI) to null left ventricular blood signal. The phantom experiment was carried out to evaluate the reliability of the scout scan for TI selection and to compare CNR between myocardium, blood and scar for both blood-nulling and conventional myocardium-nulling approaches. The proposed 3D grey-blood LGE sequence was compared to standard 2D grey-blood LGE in-vivo in terms of scar detection and image quality. Scar detection analysis was performed using the 17-segment ventricular model, whereas image quality score was performed using a 4-point Likert scale (1: non-diagnostic, 4: excellent diagnostic quality). 2D LGE acquisition parameters included: bSSFP readout with FA=45deg, resolution=1.4x1.4mm2, slice thickness=8mm, and 12 second breath-hold/slice. One slice in the two-chamber, three-chamber and four-chamber orientations, and 13-15 slices in the short-axis orientation were acquired in a total acquisition time of ~ 3min30sec excluding breath-hold pauses and commands. 0.15 mml/Kg of gadobutrol was used for patient scans and 2D and 3D grey-blood LGE was performed ~10min and 20-25min after contrast injection respectively.
Results
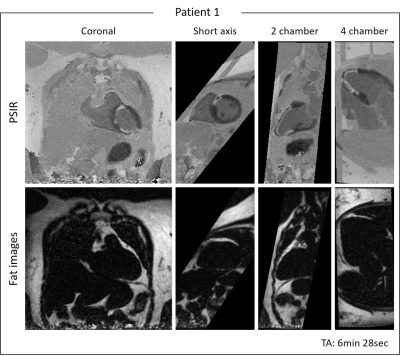
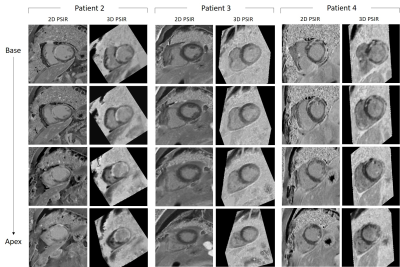
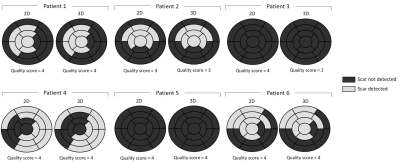
Phantom: Good nulling of each phantom vial (Fig.2A) was achieved by selecting the correct TI on the scout scan, particularly a TI=240ms and TI=395ms nulled the vial corresponding to post-contrast blood and myocardium respectively (Fig.2B). CNRmyoc-blood, CNRscar-myoc and CNRscar-blood for blood- and myocardium-nulling are shown in Fig.2C: a higher CNRscar-blood is obtained with the grey-blood approach, consistent with previously reports in the literature2.Patients: A motion-compensated 3D grey-blood LGE volume and co-registered fat volume are shown for one patient P1 in Fig.3. The 3D nature of the acquisition allowed to reformat the acquired datasets in different orientations. Good visualization of scar was observed in all reformatted views with good scar-myocardium and scar-blood contrast. The proposed 3D grey-blood LGE is qualitatively compared to standard 2D grey-blood LGE in Fig.4 for three representative patients. Good scar depiction and delineation is observed with the proposed 3D sequence, comparable to standard 2D approach for patients P2 and P4, whereas no scar was detected in patient P3. Furthermore, the contrast between myocardium, blood and scar obtained with the proposed 3D grey-blood LGE is comparable to that obtained with standard 2D LGE. The 2D grey-blood LGE acquisition generally resulted in a better scar sharpness and delineation due to higher in-plane image resolution (1.4mm vs 2mm), nevertheless excellent agreement in scar detection and comparable image quality scores were obtained between the 2D and 3D LGE approaches (Fig.5).
Conclusion
The proposed 3D motion-compensated grey-blood LGE sequence enables scar visualization and complementary 3D fat images in ~6min. Good CNR between myocardium, scar and blood was obtained in phantom acquisition. Good agreement in scar visualization and detection was observed between the proposed approach and standard 2D grey-blood LGE imaging in patients. Future work will investigate the increase in spatial resolution to improve scar visualization and validation in a larger cohort of patients.Acknowledgements
This work was supported by EPSRC (EP/L015226/1, EP/P001009/1, EP/P007619 and EP/P032311/1) and the Wellcome EPSRC Centre for Medical Engineering (NS/A000049/1).References
1. Kellman, P. et al. Dark blood late enhancement imaging. J. Cardiovasc. Magn. Reson. 18, 77 (2016).
2. Holtackers, R. J., Chiribiri, A., Schneider, T., Higgins, D. M. & Botnar, R. M. Dark-blood late gadolinium enhancement without additional magnetization preparation. J. Cardiovasc. Magn. Reson. 19, 64 (2017).
3. Kellman, P. & Arai, A. E. Cardiac imaging techniques for physicians: late enhancement. J. Magn. Reson. Imaging 36, 529–542 (2012).
4. Prieto, C. et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J. Magn. Reson. Imaging 41, (2015).
5. Bustin, A. et al. Five-minute whole-heart coronary MRA with sub-millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D-PROST reconstruction. Magn. Reson. Med. 0, (2018).
6. Henningsson, M. et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn. Reson. Med. 67, (2012).
7. Pruessmann, K. P., Weiger, M., Börnert, P. & Boesiger, P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn. Reson. Med. 46, 638–651 (2001).
8. Liu, J., Peters, D. C. & Drangova, M. Method of B0 mapping with magnitude-based correction for bipolar two-point Dixon cardiac MRI. Magn. Reson. Med. 78, 1862–1869 (2016).
Figures