2042
Kinetics of O-17-labeled water in the brain parenchyma and cerebrospinal fluid of aquaporin-4-knockout mice on indirect MRI
Yutaka Hoshino1, Hiroyuki Kameda1, Taisuke Harada1, Yuji Komaki2, Tomoe Ishikawa3, Masato Yasui3, and Kohsuke Kudo1
1Department of Diagnostic and Interventional Radiology, Hokkaido University Hospital, Sapporo, Japan, 2Live Imaging Center, Central Institute for Experimental Animals, Kawasaki, Japan, 3Department of Pharmacology, Keio University School of Medicine, Tokyo, Japan
1Department of Diagnostic and Interventional Radiology, Hokkaido University Hospital, Sapporo, Japan, 2Live Imaging Center, Central Institute for Experimental Animals, Kawasaki, Japan, 3Department of Pharmacology, Keio University School of Medicine, Tokyo, Japan
Synopsis
We evaluated the water kinetics of the brain of intoxication model mice to
compare aquaporin-4-knockout (AQP4-KO) and wild-type (WT) control mice, using
T2-weighted indirect MRI with 17O-labeled water. Eleven AQP4-KO and
eight WT mice were scanned with 7T MRI using the RARE sequence. 17O-labeled
water was administered intraperitoneally. The whole scanning took 540 phases
and 45 min, and signals were converted to 17O concentrations. In ROIs
of the brain parenchyma, steady-state 17O concentrations were
slightly higher in AQP4-KO than in WT. The latest imaging method revealed that the
deficiency of AQP4 might lead to water accumulation in the paravascular space.
Purpose
About 60 % of the human body is composed of water, which is essential for life. In the brain, water plays a vital role. Water kinetics of the human body are regulated by aquaporins, particularly by aquaporin-4 (AQP4) in the brain. Dysfunctions of AQP4 lead to brain diseases, such as neuromyelitis optica [1], brain edema [2], Alzheimer’s disease [3], and vascular dementia [4]. Brain edema may be cellular or vasogenic. Geoffrey T et al. [2] reported water intoxication-induced cellular edema and its decreased degree in AQP4-knockout (AQP4-KO) mice. Water kinetics could elucidate the pathophysiological mechanism of brain diseases, although still unclear.Magnetic resonance imaging (MRI) is a non-invasive and high-resolution imaging modality for the brain in both research and clinical settings. Recently, 17O-labeled water was developed as a new contrast agent owing to its advantages of safety and free diffusion [5,6]. Its application can be direct or indirect. The direct method requires specific hardware to detect MRI signals from the nucleus of 17O but has the disadvantage of high cost. Indirect MRI is used to detect 17O-induced proton signals because the current clinical MRI machines can be diverting.
In this study, we performed T2-weighted indirect MRI with 17O-labeled water, which is the latest imaging modality, to evaluate the water kinetics of the brain of the intoxication model, or AQP4-KO, mice and elucidate the role of AQP4.
Materials and Methods
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee and were performed in accordance with the institutional guidelines for animal experiments. Eleven AQP4-KO and eight wild-type (WT) control mice (C57BL6) were scanned with 7T MRI (Bruker BioSpin MRI GmbH) using the Rapid Acquisition with Relaxation Enhancement (RARE) sequence. The scanning conditions were: repetition time, 5000 ms; echo time, 88.09 ms; RARE factor, 64; field of view, 19.2 mm; matrix, 128 × 128; slice thickness, 1.0 mm; number of slices, 9; number of excitation, 1; single scan time, 5 s; number of dynamic phases, 540; total scan time, 45 min. The animals were sedated with isoflurane via inhalation. 5 min after the scanning started, 10% 17O-labeled distilled water (0.1 mL/g) with desmopressin (0.4 µg/kg) was administered intraperitoneally.Postprocessing of the imaging data was performed using in-house software (Perfusion Mismatch Analyzer: PMA) [7]. ROIs were manually placed in the basal ganglia, cerebral parenchyma, midbrain (two parts), lateral ventricle (two parts) and 3rd ventricle (Fig. 1). Signals in ROIs were converted to 17O concentrations. The acquired 17O concentrations versus time curves were averaged every 2 min and processed data were compared between AQP4-KO and WT mice using the independent t-test. P < 0.05 represented a statistically significant difference.
Results
Fig. 2 shows the time-curves of 17O concentrations of AQP4-KO and WT mice. After intraperitoneal administration of 17O-labeled water, concentrations began increasing rapidly, peaked, gradually decreased, and reached a steady state. The 17O concentrations in five representative phases were analyzed with the independent t-test between AQP4-KO and WT mice (Fig. 3).In (a) basal ganglia, (b) cerebral parenchyma and (c) midbrain, AQP4-KO showed slightly higher concentrations in latter phases than WT did. In both (d) lateral ventricle 1 and (f) 3rd ventricle, similar curve trends with (a), (b) and (c) were seen, but standard deviations of them were much larger. In (e) lateral ventricle 2, curve trend was opposite and standard deviations were much larger as well.
Discussion
In this study, the water kinetics of the brain of intoxication model mice was compared using indirect MRI with 17O-labeled water between AQP4-KO and WT mice. There were significant differences in the steady-state phases of ROIs in the brain parenchyma.The intraperitoneally administered 17O-labeled water entered the abdominal capillaries and reached the brain vessels via systemic circulation. In the brain capillaries, some water molecules passed the vascular endothelial cell gap and entered the paravascular space. Subsequently, the water molecules crossed AQP4 in the blood-brain barrier formed by the astrocyte end-feet.
There was accumulation of 17O-labeled water molecules in the ROIs of the brain parenchyma. A previous study on intoxication model mice [2] reported that brain edema decreased in AQP4-KO mice, implying that water did not reach the brain parenchyma and that water might accumulate in spaces other than the intravascular and parenchymal spaces. Previous studies reported expansion of the extracellular space in AQP4-KO mice [8] and increase perivascular space with less expression of AQP4 in the vascular dementia model mice [3]. This suggests that water might accumulate in the paravascular space.
However, the results for the ventricles are the limitation of this study. Variations in 17O concentrations were large on 2D MRI, as 1.0-mm slice thickness could cause a partial volume effect. This study revealed sufficient temporal resolution. Higher spatial resolution and lower temporal resolutions are required for precise measurements.
Conclusion
The water kinetics of the brain of AQP4-KO mice on indirect MRI with 17O-labeled water shows that the deficiency of AQP4 might lead to water accumulation in the paravascular space. The latest imaging modality should be further developed to elucidate the pathophysiological mechanism of brain diseases.Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.References
[1] Lennon VA et al., IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. The Journal of Experimental Medicine 2005; 16(4): 340-350.[2] Geoffrey T et al., Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nature Medicine 2000; 6(2): 159-163.
[3] Tarasoff-Conway JM et al., Clearance systems in the brain-implications for Alzheimer disease. Nature Review Neurology 2015; 11(8): 457-470.
[4] Venkat P et al., White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiology of Aging 2017; 50: 96-106.
[5] Gordji-Nejad A et al., Characterizing cerebral oxygen metabolism employing oxygen-17 MRI/MRS at high fields. MAGMA 2014; 27(1): 81-93.
[6] Kudo K et al., Indirect Proton MR Imaging and Kinetic Analysis of 17O-Labeled Water Tracer in the Brain. Magnetic Resonance in Medical Sciences 2018; 17(3): 223-230.
[7] Kudo K et al., Accuracy and Reliability Assessment of CT and MR Perfusion Analysis Software Using a Digital Phantom. Radiology 2013; 267(1): 201-211.
[8] Yao X et al., Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. The Journal of Neuroscience 2008; 28(21): 5460-5464.
Figures
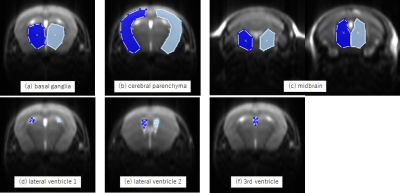
Figure 1. Regions of interest (ROIs) on the coronal slices
ROIs were placed manually on each mouse. Anatomical sites were: (a) basal ganglia, (b) cerebral parenchyma, (c) midbrain, (d) and (e) lateral ventricles, and (f) 3rd ventricle.
ROIs were placed manually on each mouse. Anatomical sites were: (a) basal ganglia, (b) cerebral parenchyma, (c) midbrain, (d) and (e) lateral ventricles, and (f) 3rd ventricle.

Figure 2. 17O concentration versus time plots for each ROI.
The 17O concentration (×10-3 %) for ROIs versus time (s) are presented. The blue and red lines show AQP4-KO and WT mice data, respectively, averaged 2 min. The error bars represent the standard deviation for the averaged points. The natural abundance of 17O, which is baseline, is 37 × 10-3 (%).
The 17O concentration (×10-3 %) for ROIs versus time (s) are presented. The blue and red lines show AQP4-KO and WT mice data, respectively, averaged 2 min. The error bars represent the standard deviation for the averaged points. The natural abundance of 17O, which is baseline, is 37 × 10-3 (%).
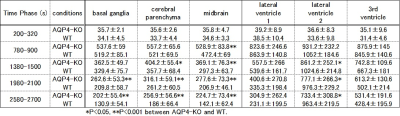
Figure 3. Averaged data of 17O concentrations in the representative phases of each ROI
Averaged 17O concentrations in five, representative time-phases of each ROI are shown. The error is a standard deviation.
* P < 0.05 and **P < 0.001
Averaged 17O concentrations in five, representative time-phases of each ROI are shown. The error is a standard deviation.
* P < 0.05 and **P < 0.001