2040
Hippocampal and Systemic Oxidative Stress in a rat model of Chronic Hepatic Encephalopathy, a multimodal approach1CIBM / LIFMET / EPFL, Lausanne, Switzerland, 2LPMC / EPFL, Lausanne, Switzerland, 3Center for Liver Disease in Children,Department of Paediatrics, HUG, Geneva, Switzerland, 4CIBM / EPFL, Lausanne, Switzerland, 5Service of Clinical Chemistry, CHUV, Lausanne, Switzerland
Synopsis
Chronic hepatic encephalopathy (CHE) is a multifactorial disease. The presence of central nervous system (CNS) and systemic oxidative stress (OS) is thought to contribute to the pathogenesis of CHE. Clinically, it is known that peripheral inflammation contributes to acute worsening of patients with CHE. We used in-vivo 1H-MRS, ex-vivo/in-vitro ESR and histology of CNS to investigate longitudinally the course of OS in the rat model of CHE. Our studies revealed antioxidant system impairment (decrease of CNS Asc and GSH, post-BDL) and increased CNS and systemic OS over the course of CHE progression, concomitant with CNS Gln and systemic NH4+ increase.
PURPOSE
The central nervous system(CNS) has a very active-oxidative-metabolism compared with other organs1,2.Physiological levels of reactive-oxygen-species(ROS) act as cellular secondary messengers. When in excess, become neurotoxic and are involved in neurodegeneration. The presence of CNS and systemic-OS contribute to the pathogenesis of CHE3. Moreover,peripheral-inflammation and systemic-OS lead to acute worsening of CHE patients4.Impaired ammonium clearance by the diseased liver leads to brain glutamine(Gln) accumulation in CHE, inducing in-vitro-ROS generation associated with astrocyte deterioration5,6.In addition,hyperammonemia impairs neutrophils function by decreasing phagocytosis and increasing spontaneous-oxidative-burst7. In a rat-model of CHE(bile-duct-ligated,BDL-rat) we have demonstrated by 1H-MRS the indirect presence of OS(i.e. decrease of brain ascorbate(Asc) and glutathione(GSH) in hippocampus, together with the early increase in plasma ammonium and brain Gln,suggestive of oxidative/osmotic stress8,9.Furthermore we have validated the OS findings using for the first time ex-vivo-ESR at a late-time-point in disease evolution10. The novelty of the present study relies on the longitudinal assessment of hippocampal and systemic-OS using a multimodal approach:in-vivo 1H-MRS followed by ex-vivo/in-vitro-ESR detection of ROS and histological measures.METHODS
In-vivo 1H-MRSA new group of Wistar male-adult-rats was BDL or sham-operated and measured before BDL(n=23) and after every 2-weeks up to 8-weeks(n=7).In-vivo 1H-MRS (9.4T,Varian/Magnex-Scientific) was used to analyze neurometabolism including antioxidant concentrations in the hippocampus(2x2.8x2mm3) using SPECIAL-sequence11(TE=2.8ms,TR=4sec;160averages).Metabolite concentrations were calculated by LCModel using water as reference.
Ex-vivo/in-vitro-ESR
ESP300E(Bruker-BioSpin,TE102-cavity) was used to measure the concentration and time-course of ROS in hippocampus and blood.The hippocampus was extracted/weighed/sliced and transferred into RPMI1640-medium(37oC) with 10mM-CMH-cell-permeable non-toxic spin-trap(Noxygen-GmbH). Blood(1mL) was collected and CMH-spin-trap was added(10mM final-concentration).After each incubation-time, tissue-suspension/blood was transferred into quartz-capillary and measured.
Neurohistology-Golgi-Cox-method
Golgi-Cox-staining uses the principle of metallic-impregnation of neurons and it was applied to reveal detailed neuronal morphology of the hippocampus.110μm-thick brain sections were cut sagittally(25 slides/hemisphere,n=8).
Lymphocytes/Polymorphonuclear cells (PMNs) count
Sterile-blood was obtained from heart(n=20). 7ml of anticoagulated-blood was used for each isolation procedure. Acridine-Orange-assay was applied to assess the lymphocytes and PMNs concentrations.
RESULTS AND DISCUSSION
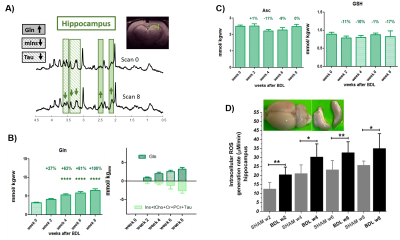
The presence of chronic liver disease was confirmed by increased plasma ammonium and bilirubin(2-weeks-post-BDL).1H-MRS revealed the well-known ammonium-induced increase in Gln(+67%,4-weeks-post-BDL) together with the decrease in brain osmolytes(Fig1A-B). Astrocytes are essential to detoxify ammonium12,while Gln increase is known to produce OS response in cells13.In this context,we have previously shown a global atrophy of astrocytes in BDL-rats8,9,suggesting an astrocytic activation and synaptic depression induction14.
The main antioxidants measured by 1H-MRS showed a decrease at 4-weeks-post-BDL:Asc -11%, GSH -10%, reaching -17% at 8-weeks(Fig1C). Of note, the previously observed Asc decrease at 8-weeks-post-BDL was absent, probably due to the smaller number of rats.The hippocampus ex-vivo-ESR revealed a significant increase of OS +68%(p<0.01) already at 2-weeks-post-BDL,thereby enhancing the time-resolution and understanding of neurometabolic changes in early CHE relative to 1H-MRS.
ROS as signaling and stress-molecules affect synaptic-plasticity and are critical for long-term-potentiation(LTP) in the hippocampus,,a form of synaptic-plasticity for learning/memory but are also involved in aging-related-impairment the long-term-depression(LTD)15,16.Dismutation of superoxide-anion generates H2O2 in hippocampal neurons, affecting LTP in complex ways.The excess of ROS attenuates LTP and synaptic neurotransmission17,18.
In-vitro-ESR on peripheral-blood demonstrated a significant increase of systemic OS(+49%,6-weeks-post-BDL).These results are consistent with the increase in the peripheral white-blood-cells count: lymphocytes:+177%,PMNs:+78% at 2-weeks-post-BDL. Ammonia induced PMNs swelling7 was detected already at 2-weeks-post-BDL.This is a sign of impaired phagocytosis and increased prevalence of spontaneous-oxidative-burst7(Fig.2D).This finding may be in keeping with the known vulnerability to infection in cirrhosis and the consequent risk for HE decompensation.
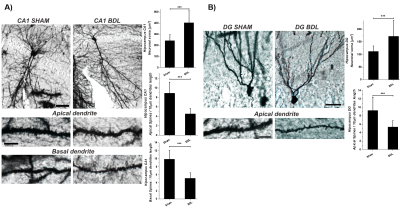
The Golgi-Cox-staining showed a significant increase in CA1(~67%, p<0.001) and DG(~54%,p<0.001) neuronal-soma surface and a significant loss of dendritic-spines-density in CA1~49%(apical,basal,p<0.001) and DG~43%(apical,p<0.001) in hippocampal neurons(Fig.3).It’s known that different neurons have different levels of vulnerability to OS.The hippocampus,amygdala,and cerebellar granule cells have been reported as the most susceptible and therefore may the first to exhibit functional decline19.In addition NAA(showing -9% decrease by 1H-MRS) may be a minor contributor to neuronal volume-regulation which responds to hypo-osmolarity and acts as a co-transit substrate for a putative molecular-water-pump to remove excess of water from neurons20. Furthermore, the hippocampus plays a critical role in several higher brain functions, such as learning/memory, spatial-encoding and is one of the few brain regions which exhibits adult-neurogenesis21.The alterations of hippocampus neuronal morphology due to metabolic-degenerative-disorders are often associated with cognitive impairment, suggesting that the hippocampus may be an interesting area to study in HE.
CONCLUSIONS
The present study shows the potential of multidisciplinary-approach to monitor longitudinally central and systemic-OS in a rat-model of CHE using in-vivo 1H-MRS combined with ex-vivo/in-vitro-ESR spectroscopy. The presence of hippocampal and systemic-OS was measured early,i.e.2-weeks post-BDL. Moreover, the 1H-MRS findings were validated by ex-vivo-ESR. Our previously depicted astroglia atrophy8,9 may cause reduction in network connectivity, synaptic coverage and homeostatic capabilities24.These results corroborate with the present Golgi-Cox-staining showing significant increase of hippocampal neuronal soma surface and dendritic-spines-density loss.Therefore, it’s tempting to extrapolate that high OS levels are associated with a loss of neurons connectivity associated with behavioral deterioration in a rat-model of CHE.Acknowledgements
Supported by CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations and the SNSF project no 310030_173222/1References
1Halliwell B. J Neurochem 59: 1609–1623,1992;2 Samina Salim, J Pharmacol Exp Ther 360:201–205, 2017; 3Bosoi CR, Rose CF. Metab Brain Dis. 2013 Jun;28(2):175-8;4Cristina R. Bosoi, Christopher F. Rose, Metabolic Brain Disease,2012; 5Skowronska M. and Albrecht J., Neurochemistry International, 2012;6Lachman V. et al., Archives of Biochemistry and Biophysics, 2013; 7Debbie L. Shawcross et al., HEPATOLOGY, Vol. 48, No. 4, 2008; 8K. Pierzchala et al., ISMRM2019 # 2279; 9Olivier Braissant, et al.,Journal of Hepatology 2019; 10K. Pierzchala et al., ISMRM2018 # 448; 11Mlynárik et al, Magn Reson Med 2006; 12Gavin A. K. et al., Liver Int. 2014: 34: 1184–1191; 13Jan Albrecht and Michael D. Norenberg, HEPATOLOGY 2006;44:788-794; 14Rogier Min & Thomas Nevian, 2012 nature NEUROSCIENENCE; 15Lauren T. Knapp and Eric Klann, Journal of Neuroscience Research, 2002;16Cynthia A.et al., PNAS 2009 August, 106 (32) 13576-13581; 17Mol Neurobiol(2017); 18Xinkun Wang and Elias K. Michaelis,Frontiers in Aging Neuroscience, 2010; 19Wang X and Michaelis EK, Front Aging Neurosci 2:12(2010); 20Brian Ross et al.,Progress in Neurobiology 81 (2007); 21Peter Jonas and John Lisman, Frontiers in Neural Circuits, September 2014; 22Bruna Bellaver et al.,Mol Neurobiol,2017;23Cecilia Hidalgo and Alejandra Arias-Cavieres PHYSIOLOGY • Volume 31 • May 2016; 24Alexei Verkhratsky, José J. Rodríguez, and Luca Steardo, The Neuroscientist 2014.Figures
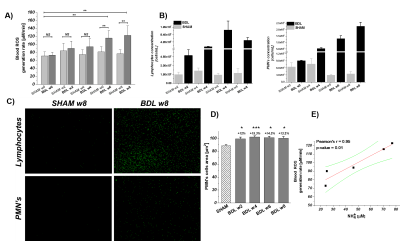
A) Longitudinal evolution of systemic OS: week 0 before BDL to 8 weeks post BDL. B) Immune system response to CHE marked by an increase in inflammatory cells (lymphocytes/PMNs). C) Acridine Orange stained lymphocytes/PMNs nuclei shows significant increase of cells number. D) Ammonia induced PMNs dysfunction demonstrates as an increase of cell size. E) Pearson correlation shows a strong correlation r=96 (p=0.01) between the ROS generation and the ammonia concentration. Data presented as mean ± SD (One way Anova with post-hoc Tukey HSD): *p<0.05,**p<0.01,***p<0.001,****p<0.0001.