2034
Clinical risk factors for smoking-related neurobiological damage1Neurosciences Research Centre, Molecular and Clinical Sciences Research Institute, St George's University of London, London, United Kingdom, 2Institute for Infection and Immunity, St George's University of London, London, United Kingdom, 3Respiratory Medicine, St George's University Hospital NHS Foundation Trust, London, United Kingdom
Synopsis
Elderly cigarette smokers have an elevated risk of cognitive decline and neurological disease, however, the pathophysiological mechanisms are unclear. This study investigated which biological factors are responsible. 100 participants (age: 68±8 years, 69% male) with significant smoking history and range of respiratory and cardiovascular disease were recruited. Multiple linear regression showed that higher blood pressure, reduced respiratory function, hypoperfusion and biomarkers of cardiac (troponin T) damage and systemic inflammation (C-reactive protein) were associated with brain magnetic resonance markers of neurobiological damage and there may be complex interactions between them. Results support a vascular aetiology with contributions from systemic inflammation.
INTRODUCTION
Elderly cigarette smokers have an elevated risk of cognitive decline and neurological disease.1 Previous magnetic resonance (MR) studies show that smoking is associated with progression of white matter hyperintensities (WMHs), 2 global and local reductions in cortical and subcortical grey matter3 and microstructural abnormalities.4 The neuropathological mechanisms are unclear, however smoking has known adverse effects on the cardiovascular system, causes systemic inflammation and oxidative stress5-7 and leads to medical conditions including Chronic Obstructive Pulmonary Disease (COPD) and Coronary Artery Disease, which are themselves linked with cognitive impairment and brain change.8,9 This study investigated the relationship between biological risk factors and neurobiological damage in an elderly cohort with significant history of smoking and range of airflow limitation and cardiovascular disease.METHODS
100 participants with significant smoking history (age: 68±8 years, 69% male, >10 pack years smoking). Demographic, clinical and biochemical markers assessing respiratory function, oxygen saturations, blood pressure, aortic stiffness (pulse wave velocity and augmentation index), breathlessness, COPD disease status, psychological status, cognitive impairment, systemic inflammation (high-sensitivity C-reactive protein, hs-CRP, fibrinogen, neutrophils) and cardiac damage (troponin T) were acquired. Retinal fundus photography provided measures of retinal microcirculation. Whole-brain T1-weighted (T1W), Fluid-attenuated Inversion Recovery (FLAIR), Diffusion Tensor Imaging (DTI) and pseudo-continuous arterial spin labelling (pCASL) scans were acquired using a 3-Tesla Phillips Achieva dual TX MR system. Measures of cerebral atrophy, WMH volume, white matter microstructural damage (fractional anisotropy and mean diffusivity) and cerebral blood flow (CBF) were computed. All analyses controlled for age and sex. Principal component analysis was used to reduce brain, retinal, cardiovascular, and respiratory variables into components. Relationships between clinical measures and brain variables and components were tested using Pearson’s correlations and multiple linear regression. Multivariate relationships were plotted on three-dimensional polynomial surface diagrams.RESULTS
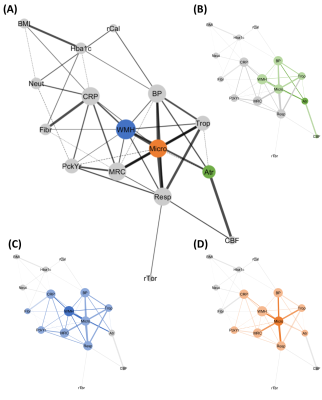
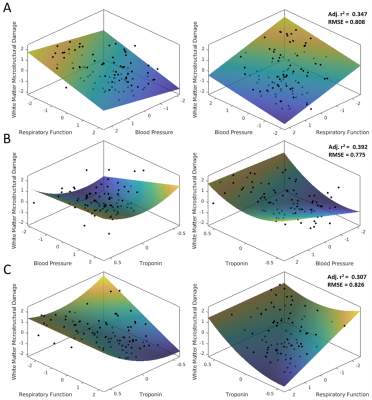
Principal components representing cerebral atrophy, white matter microstructural damage, retinal vessel calibre, retinal vessel tortuosity, respiratory function and blood pressure were extracted. Inflammatory markers did not form a component. A large number of correlations were found between clinical and brain MR measures (Figures 1 and 2). Multiple linear regression showed that higher blood pressure (p=0.013), troponin T (p=0.041) and lower respiratory function (p=0.010) were independent predictors of greater white matter microstructural damage. Higher hs-CRP independently predicted WMH volume (p=0.004) and lower CBF (p<0.001) independently predicted cerebral atrophy. Multivariate relationships are shown in Figure 3. High blood pressure and low lung function appeared to have an additive effect on white matter microstructural damage. Whereas high blood pressure and high troponin were individually associated with greater white matter microstructural damage but did not have an additive effect. Similarly, high troponin and low respiratory function individually were associated with greater white matter microstructural damage but did not have an additive effect.DISCUSSION
Independent associations between higher blood pressure, higher troponin T, lower respiratory function and greater white matter microstructural damage are consistent with a vascular aetiology for smoking-related neurobiological decline. Cigarette smoking and hypertension are established risk factors for cardiovascular disease.10,11 Associations have also been reported between raised blood pressure, cardiac troponins, reduced respiratory function (or presence of COPD) and several MR markers of cerebral small vessel disease (CSVD).12-14 Prolonged hypertension promotes vessel wall remodelling and endothelial dysfunction, 15 compromising autoregulation and leaving the brain vulnerable to hypoperfusion or oxygen desaturation e.g. due to lung disease.15 This is consistent with this study's finding of an additive effect of high blood pressure and reduced respiratory function on white matter microstructural damage. Chronic low-level troponin release may be symptomatic of diffuse subclinical small vessel heart disease, 16 suggesting vascular disease may be affecting multiple end-organs.17The independent association between hs-CRP and WMH volume is consistent with previous studies showing relationships between serum CRP or upregulation of inflammation-related genes, and markers of CSVD.18,19 It is presently unknown whether systemic inflammation has an aetiological role in CSVD or is an epiphenomenon.
The independent association between lower CBF and cerebral atrophy adds to a large body of evidence showing the adverse effects of reduced CBF on brain structure.20,21 This study’s cross-sectional design does not allow the direction of causality to be determined. However, global22 and regional reductions in CBF are known to occur in chronic smokers, 23 with proposed mechanisms including smoking-induced oxidative stress and endothelial dysfunction, nitric oxide vasodilatation, neurovascular coupling, hypocapnia, and autoregulatory dysfunction.23 Furthermore, aberrant vascular autoregulation, for example, as a result of CSVD or atherosclerosis is likely to exacerbate hypoperfusion and cerebral atrophy.
CONCLUSION
This study provided a cross-sectional investigation of the relationship between a range of biological risk factors for neurobiological damage in an elderly cohort with significant history of cigarette smoking and range of airflow limitation and cardiovascular disease. Higher blood pressure, serum troponin T and lower respiratory function appear to be independent and possibly additive risk factors for white matter microstructural damage. Associations were also found between higher serum hs-CRP and greater WMH volume and between lower CBF function and cerebral atrophy. This suggests that multiple preventative or therapeutic interventions are required to target a range of pathophysiological mechanisms in any attempts to reduce neurobiological decline and the development of cognitive impairment people with a history of smoking.Acknowledgements
This study was partially funded as part of a National Institute for Health Research (NIHR) clinical doctoral fellowship: DRF-2016-09-088.References
1. Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a Risk Factor for Dementia and Cognitive Decline: A Meta-Analysis of Prospective Studies. Am. J. Epidemiol. 2007;166:367–378.
2. van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences: Rotterdam Scan Study. Stroke 2008;39:2712–2719.
3. Pan P, Shi H, Zhong J, et al. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2013;34:813–817.
4. Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. NeuroImage 2011;54:42–48.
5. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004;43:1731–1737.
6. Çolak Y, Afzal S, Lange P, Nordestgaard BG. Smoking, Systemic Inflammation, and Airflow Limitation: A Mendelian Randomization Analysis of 98 085 Individuals From the General Population. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2019;21:1036–1044.
7. Zuo L, He F, Sergakis GG, et al. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014;307:L205–L218.
8. Burkauskas J, Lang P, Bunevičius A, Neverauskas J, Bučiūtė-Jankauskienė M, Mickuvienė N. Cognitive function in patients with coronary artery disease: A literature review. J. Int. Med. Res. 2018;46:4019–4031.
9. Spilling CA, Bajaj M-PK, Burrage DR, et al. Contributions of cardiovascular risk and smoking to chronic obstructive pulmonary disease (COPD)-related changes in brain structure and function. Int. J. COPD 2019;14:1855-1866.
10. Whitmer R, Sidney S, Selby J, Johnston S, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281.
11. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666–c3666.
12. von Rennenberg R, Siegerink B, Ganeshan R, et al. High-sensitivity cardiac troponin T and severity of cerebral white matter lesions in patients with acute ischemic stroke. J. Neurol. 2019;266:37–45.
13. Dadu RT, Fornage M, Virani SS, et al. Cardiovascular Biomarkers and Subclinical Brain Disease in the Atherosclerosis Risk in Communities (ARIC) Study. Stroke J. Cereb. Circ. 2013;44:1803–1808.
14. Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 2005;237:251–257.
15. Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr. Hypertens. Rep. 2017;19:24.
16. Moreno V, Hernández-Romero D, Vilchez JA, et al. Serum Levels of High-Sensitivity Troponin T: A Novel Marker for Cardiac Remodeling in Hypertrophic Cardiomyopathy. J. Card. Fail. 2010;16:950–956.
17. Berry C, Sidik N, Pereira AC, et al. Small‐Vessel Disease in the Heart and Brain: Current Knowledge, Unmet Therapeutic Need, and Future Directions. J. Am. Heart Assoc. 2019.
18. Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: Contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2012;1822:361–369.
19. Fornage M, Chiang YA, O’Meara ES, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke J. Cereb. Circ. 2008;39:1952–1959.
20. Alosco ML, Gunstad J, Jerskey BA, et al. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav. 2013;3:626–636.
21. Austin BP, Nair VA, Meier TB, et al. Effects of Hypoperfusion in Alzheimer’s Disease. J. Alzheimers Dis. 2011;26:123–133.
22. Meyer JS, Rauch G, Rauch RA, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiol. Aging 2000;21:161–169.
23. Elbejjani M, Auer R, Dolui S, et al. Cigarette smoking and cerebral blood flow in a cohort of middle-aged adults. J. Cereb. Blood Flow Metab. 2019;39:1247–1257.
Figures