2032
Structural covariance differences in mild and refractory mesial temporal lobe epilepsy1Neuroscience Research Center, University "Magna Graecia", Catanzaro, Italy, 2Institute of Neurology, University "Magna Graecia", Catanzaro, Italy
Synopsis
Mesial Temporal Lobe Epilepsy (MTLE), the most common form of adult-onset epilepsy, represents a convenient candidate to study brain structural covariance networks. In particular, mild MTLE is a precious resource to study brain structure and function independently from the damage caused by seizure recurrence and medication use. In this work, we used graph analysis to investigate structural covariance networks in mild and refractory MTLE, and found that while the latter shows altered integration and segregation network properties, mMTLE has graph-based characteristics suggestive of a possibly inborn different brain organization that might act as protective factor against seizure recurrence.
Introduction
In health and pathology, there are marked inter-individual differences in the structure of cortical regions. In particular, between-subject variability is much larger when considering the volume of a given structure compared to whole-brain volume [1]. The phenomenon known as 'structural covariance' shows that inter-individual differences in regional structure are coordinated within communities of brain regions, which fluctuate together (i.e., co-vary) in size across the population. Networks of structural covariance have been studied in drug-resistant temporal lobe epilepsy, especially since increasing evidence supports the hypothesis that specific cortical and subcortical networks play a fundamental role in the genesis and expression of seizures [2]. However, little is known regarding possible alterations of structural co-variance patterns in patients with mild mesial temporal lobe epilepsy (mMTLE). The main features of this phenotype include seizure onset in adulthood, unremarkable past medical history, viscero-sensory auras, and long-term seizure freedom (>24 months) with or without antiepileptic medication [3]. Approximately one third of patients with mMTLE have MRI evidence of hippocampal sclerosis (HS), which was previously considered a hallmark of refractoriness. Very recently, it has been shown that mMTLE patients with HS possess 3-times higher likelihood of becoming refractory later on in life than those without HS [4]. Mild MTLE represents a superb resource to better delineate the biological substrates underlying MTLE, in the search for correlates of the epileptic syndrome itself, and in this study we aimed at evaluating its characteristics in terms of structural covariance using the graph theory approach [5].Methods
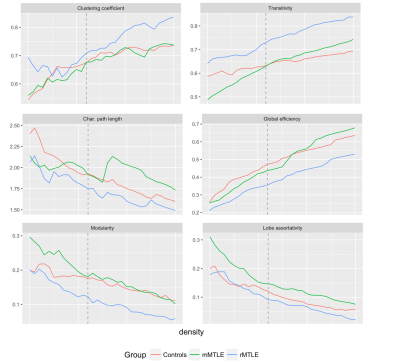
Sixty-four patients with mMTLE (35 female, mean age (standard deviation) 37.0 (9.1)), 47 patients with refractory MTLE (rMTLE; 25 female, mean age (standard deviation) 40.3 (9.7)) and 114 healthy controls (HC; 58 female, mean age (standard deviation) 38.7 (11.1)) underwent the same 3 Tesla MRI protocol including whole-brain, 3D T1-weighted, spoiled gradient recall echo (TE/TR = 3.7/ 9.2 ms, flip angle 12°, voxel size= 1×1×1 mm3). All T1 images were analyzed using FreeSurfer 5.3. Cortical thickness measures were extracted for 34 grey matter regions per hemisphere (68 total). Quality control of the parcellation was conducted following standardized ENIGMA protocols (http://enigma.usc.edu). First, model residuals were generated and correlation matrices were created over a set of densities (0.05-0.40, steps of 0.01) using the brainGraph package available in R. Structural covariance networks were estimated using graph theory, and the following variables were compared across groups: clustering coefficient, measuring how strongly inter-connected neighboring nodes are; characteristic path length and global efficiency, related to the global integration of the network; modularity and lobe assortativity, which measure network segregation; transitivity, a variant of the clustering coefficient not influenced by nodes with a low degree (i.e., with less importance in the network). All measures were compared through analysis of covariance, with age, sex and intracranial volume as covariates. Statistical significance threshold was set at p=0.05, after correction for multiple comparisons performed using Tukey Honest Significant Difference and False Discovery Rate methods.Results
Patients with rMTLE showed abnormalities in network segregation and integration properties (Figure 1). At a chosen density of 0.22 (lowest density for which 95% of vertices were connected for all groups), rMTLE showed significantly lower values of global efficiency, path length and modularity compared to both mMTLE and HC. Clustering coefficient and transitivity, instead, were significantly higher in rMTLE compared to both other groups (all corrected p-values < 0.05). Interestingly, lobe assortativity was higher in mMTLE patients compared to rMTLE and HC.Discussion
Structural covariance networks characteristics were widely altered in patients with refractory MTLE, but not in patients with mild MTLE. Since presence of HS was equally distributed across groups, differences in graph metrics might not be driven by temporal lobe abnormalities, but rather complement them. Patients with rMTLE showed modification of both integration and segregation properties of the networks, suggesting that the recurrence of seizures and the use of multiple antiepileptic drugs might have a disruptive effect on the structural organization of the entire brain. Higher lobe assortativity indicates a higher ratio between inter-lobar and intra-lobar correlation: this finding in mMTLE might be the result of pre-existing, possibly congenital, different structural covariance relationship between brain regions compared to refractory patients, which may ultimately lead to a more benign response to the insurgence of the epileptic syndrome.Conclusions
These findings further corroborate that epilepsy should be considered as a network rather than focal disease. Further studies on the different organization of structural covariance networks in mild and refractory MTLE might shed new insights onto the etiology of the two syndromes and their different prognosis.Acknowledgements
No acknowledgement found.References
[1] Alexander-Bloch et al. Imaging structural co-variance between human brain regions. Nat Rev Neuroscience 2013; doi: 10.1038/nrn3465.
[2] Bernhardt BC, Hong S, Bernasconi A and Bernasconi N (2013) Imaging structural and functional brain networks in temporal lobe epilepsy. Front. Hum. Neurosci. 7:624. doi: 10.3389/fnhum.2013.00624
[3] Labate A, Gambardella A, Andermann E, et al. Benign mesial temporal lobe epilepsy. Nat Rev Neurol 2011;7:237–240.
[4] Labate A, Aguglia U, Tripepi G, et al. Long-term outcome of mild mesial temporal lobe epilepsy. Neurology 2016; doi:10.1212/WNL.0000000000002674.
[5] Bernhardt et al. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb cortex 2011; 21:2147-2157.
[6] Watson CG, Stopp C, Newburger JW, Rivkin MJ. Graph theory analysis of cortical thickness networks in adolescents with d-transposition of the great arteries. Brain and Behavior 2018. doi:10.1002/brb3.834.