2031
Self-efficacy brain: General self-efficacy mediates the link of lenticular nucleus volume to affective well-being in late adolescence1Huaxi MR Research Center (HMRRC), Chengdu, China
Synopsis
Previous literature has shown importance of positive traits to a spectrum of health outcomes, in particular to subjective well-being. General self-efficacy (GSE), a kind of motivational belief in competence with prospective and operative nature, is one such trait. Here, structural magnetic resonance imaging along with self-report tests were applied to investigate neural basis of GSE and the underlying neural mechanism of how GSE promotes subjective well-being during late adolescence. Our findings showed a positive link between GSE and the left lenticular nucleus volume and revealed a mediating role of GSE in the relation of lenticular nucleus volume with affective well-being.
Introduction
As one of the most important psychological constructs in social cognitive theory, general self-efficacy (GSE) reflects a broad confidence in one's own coping competence across a variety of situations. Previous evidence has consistently shown a positive effect of GSE on a spectrum of health outcomes, in particular on subjective well-being (SWB) [1-5]. However, less is known about the neurostructural basis of GSE and the underlying neural promoting pathway of GSE on SWB. In this work, we examined these issues in a large scale of healthy adolescents aged 16 to 20 years via structural magnetic resonance imaging (S-MRI) along with a battery of self-report tests.Methods
A total of 231 healthy right-handed local high school students (mean age = 18.48 years, standard deviation = 0.54, 52% female) were recruited in this study. The GSE scale [6, 7], the Positive and Negative Affect Schedule [8] and the Satisfaction with Life Scale [9] were respectively employed to evaluate individuals' GSE, affective and cognitive component of SWB. Additionally, the Raven’s advanced progressive matrix [10] was utilized to assess individuals’ general intelligence to eliminate the possibility that the observed correlations between brain structures and GSE were driven by general intelligence. The S-MRI scans were performed on a 3.0-T Trio Erlangen MRI (Siemens, Germany). A high-resolution T1-weighted anatomical image was acquired for each participant using a magnetization-prepared rapid gradient-echo sequence (voxel size, 1 × 1 × 1 mm3; flip angle, 9°; matrix size, 256 × 256; slice thickness, 1 mm; 176 slices; echo time, 2.26 ms; inversion time, 900 ms; repetition time, 1,900 ms). All S-MRI data were preprocessed using Statistical Parametric Mapping program (SPM12; Welcome Department of Cognitive Neurology, London, UK; http:// www.fil.ion.ucl.ac.uk/spm/) with steps below [11] to determine regional gray matter volume (rGMV): adjusted the origin of the images to the anterior commissure; segmented the images into gray matter (GM), white matter, and cerebrospinal fluid; aligned and resampled the GM images and normalized them to a study-specific template in Montreal Neurological Institute (MNI152) space using Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) in SPM12 [12]; modulated the GM values in each voxel with Jacobian determinants and smoothed the images with an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. Thus, the images representing the rGMV were obtained and adopted in the subsequent analyses. We examined brain GM correlates of GSE using a whole-brain multiple regression analysis. All the resulting maps were corrected for multiple comparisons with a voxelwise threshold of p < 0.001 combined a cluster threshold of p < 0.05 using nonstationary cluster test based on random field theory [13]. Next, we conducted prediction analyses with a popular appoach combined four-fold balanced cross-validation and linear regression [14-16] to assess the robustness of the previously identified brain-GSE connections. Finally, we performed mediation analyses [17] to explore whether the previously detected brain structures link to a certain component of SWB through GSE.Results
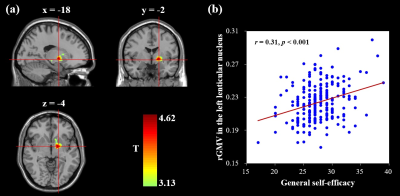
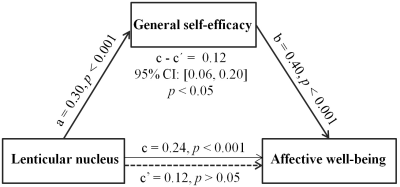
Whole-brain multiple regression analyses which adjusted for age, sex, general intelligence and total GM volume (TGMV) indicated a positive association between GSE and the rGMV of the left lenticular nucleus, an area extending from the left putamen to the left globus pallidus (MNI coordinates: -18, -2, -4; cluster size = 450 voxels; T = 4.62; see Figure 1). Prediction analyses confirmed the predictive ability of the left lenticular nucleus volume to GSE [rfinal (predicted, observed) = 0.27, p < 0.001] after controlling for age, sex, general intelligence and TGMV. Furthermore, mediation analyses that controlled for age, sex, general intelligence, and TGMV revealed a mediating role of GSE in the relation between the left lenticular nucleus and affective well-being (indirect effect, 0.12; 95% CI = [0.06, 0.20], p < 0.05; see Figure 2).Discussion
The current research investigates the neuroanatomical substrates of GSE in late adolescents and the neural promoting mechanism of how GSE impacts SWB. First, greater lenticular nucleus volume was linked to a stronger GSE. This finding fits well with a previous investigation of Nakagawa et al. (2017) [18], showing that young adults who have higher neuronal density in the lenticular nucleus have higher GSE scores. This finding is also similar to prior evidence on self regulation [19-21], which is a strongly related construct to GSE. Given lenticular nucleus is known to be a core station in reward system, that has been shown to serve a significant role in sustaining motivational functions [22-25], interindividual variation in the left lenticular nucleus volume probably bring about individual discrepancy in the motivational value, which in turn contribute to individual difference in GSE. Second, we detected a mediating role of GSE in linking left lenticular nucleus volume to affective component of SWB, suggesting a "brain - GSE - affective well being" pathway to enhance affective component of SWB.Conclusion
To conclude, the current study provides a direct evidence for neuroanatomical substrates underlying GSE in late adolescence, and suggested a potential "brain - positive traits - health outcomes" appoach for enchancing affective component of SWB.Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Nos. 81621003, 81820108018 and 31800963), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT16R52) of China, the China Postdoctoral Science Foundation (Grant No. 2019M653421), and the Postdoctoral Interdisciplinary Research Project of Sichuan University. Dr Q.G. would also like to acknowledge the support from his Changjiang Scholar Professorship Award (Award No. T2014190) of China and the American CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education, USA.References
[1] N. Azizli, B.E. Atkinson, H.M. Baughman, E.A. Giammarco. Relationships between general self-efficacy, planning for the future, and life satisfaction. Personality and Individual Differences. 2015; 82: 58-60.
[2] F.S. Çakar. The Relationship between the Self-Efficacy and Life Satisfaction of Young Adults. International Education Studies. 2012; 5(6): 123-130.
[3] A. Luszczynska, B. Gutiérrez-Doña, R. Schwarzer. General self‐efficacy in various domains of human functioning: Evidence from five countries. International journal of Psychology. 2005; 40(2): 80-89.
[4] R. Zhang. Positive affect and self-efficacy as mediators between personality and life satisfaction in Chinese college freshmen. Journal of Happiness Studies. 2016; 17(5): 2007-2021.
[5] M. Strobel, A. Tumasjan, M. Spörrle. Be yourself, believe in yourself, and be happy: Self‐efficacy as a mediator between personality factors and subjective well‐being. Scandinavian Journal of Psychology. 2011; 52(1): 43-48.
[6] Schwarzer, R., & Jerusalem, M. (1995). Generalized self-efficacy scale. In J. Weinman, S. Wright, & M. Johnston (Eds.), Measures in health psychology: A user’sportfolio. Causal and control beliefs (pp. 35–37). Windsor, UK: NFER-NELSON.
[7] R. Schwarzer, J. Bäßler, P. Kwiatek, K. Schröder, J.X. Zhang. The assessment of optimistic self‐beliefs: comparison of the German, Spanish, and Chinese versions of the general self‐efficacy scale. Applied Psychology. 1997; 46(1): 69-88.
[8] D. Watson, L.A. Clark, A. Tellegen. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988; 54(6): 1063.
[9] E. Diener, R.A. Emmons, R.J. Larsen, S. Griffin. The satisfaction with life scale. Journal of personality assessment. 1985; 49(1): 71-75.
[10] J. Raven. The Raven's progressive matrices: change and stability over culture and time. Cognitive psychology. 2000; 41(1): 1-48.
[11] J. Ashburner. VBM Tutorial. 2015; Tech. rep, Wellcome Trust Centre for Neuroimaging, London, UK.
[12] J. Ashburner. A fast diffeomorphic image registration algorithm. NeuroImage. 2007; 38(1): 95-113.
[13] S. Hayasaka, K.L. Phan, I. Liberzon, K.J. Worsley, T.E. Nichols. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004; 22(2): 676-687.
[14] J.R. Cohen, R.F. Asarnow, F.W. Sabb, R.M. Bilder, S.Y. Bookheimer, B.J. Knowlton, R.A. Poldrack. Decoding developmental differences and individual variability in response inhibition through predictive analyses across individuals. Frontiers in human neuroscience. 2010; 4: 47.
[15] T.M. Evans, J. Kochalka, T.J. Ngoon, S.S. Wu, S. Qin, C. Battista, V. Menon. Brain Structural Integrity and Intrinsic Functional Connectivity Forecast 6 Year Longitudinal Growth in Children's Numerical Abilities. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015; 35(33): 11743-50.
[16] K. Supekar, A.G. Swigart, C. Tenison, D.D. Jolles, M. Rosenberg-Lee, L. Fuchs, V. Menon. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proceedings of the National Academy of Sciences. 2013; 110(20): 8230-8235.
[17] Hayes, A. F., & Scharkow, M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychol Sci. 2013; 24(10): 1918-1927.
[18] S. Nakagawa, H. Takeuchi, Y. Taki, R. Nouchi, Y. Kotozaki, T. Shinada, T. Maruyama. A. Sekiguchi, K. Iizuka, R. Yokoyama, Lenticular nucleus correlates of general self-efficacy in young adults. Brain Structure and Function. 2017; 222(7): 3309-3318.
[19] J. Liu, J. Liang, W. Qin, J. Tian, K. Yuan, L. Bai, Y. Zhang, W. Wang, Y. Wang, Q. Li. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neuroscience letters. 2009; 460(1): 72-77.
[20] R. Marsh, J.E. Steinglass, A.J. Gerber, K.G. O’Leary. Deficient Activity in the Neural Systems That Mediate Self-regulatory Control in Bulimia Nervosa. Arch Gen Psychiatry. 2009: 66(1): 51-63.
[21] R. Marsh, H. Zhu, R.T. Schultz, G. Quackenbush, J. Royal, P. Skudlarski, B.S. Peterson. A developmental fMRI study of self‐regulatory control. Human brain mapping. 2006: 27(11): 848-863.
[22] S. Ikemoto, C. Yang, A. Tan. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behavioural Brain Research. 2015; 290: 17-31.
[23] R. Brown, G. Pluck. Negative symptoms: the'pathology'of motivation and goal-directed behaviour. Trends in neurosciences. 2000; 23(9): 412.
[24] K.S. Smith, A.J. Tindell, J.W. Aldridge, K.C. Berridge. Ventral pallidum roles in reward and motivation, Behavioural brain research. 2009; 196(2): 155-167.
[25] W. Schultz. Multiple reward signals in the brain. Nature reviews neuroscience. 2000; 1(3): 199.
Figures