2030
Quantification of contrast enhancement from FLAIR to assess meningeal inflammation in a meningoencephalitis model1Integrated Research Facility, Division of Clinical Research, National Institute of Allergy and Infectious Diseases, Frederick, MD, United States, 2Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research sponsored by the National Cancer Institute, Frederick, MD, United States, 3Center for Infectious Disease Imaging, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD, United States
Synopsis
The purpose of this study was to utilize advanced post processing analysis to assess the degree of meningeal inflammation in an animal model of Lassa virus induced meningoencephalitis. Subtraction analysis of co-registered pre- and post-contrast FLAIR images revealed various degrees of meningeal inflammation between animals. Histopathology confirmed viral meningoencephalitis. This approach can be helpful in detecting mild to severe degrees of meningeal inflammation in a variety of infectious diseases.
Introduction
Lassa fever (LF) is an acute viral hemorrhagic disease caused by Lassa virus (LASV), a pathogen known to be associated with severe disease as well as viral encephalitis and meningitis. In severe cases, neurologic complications, such as confusion, tremors, convulsions, and coma, are frequent.1 Hearing loss is also a very common long-term morbidity in survivors.2 However, the pathogenesis of neurologic and auditory complications of Lassa fever is poorly understood. The purpose of this study was to quantify central nervous system inflammation in an animal model of LASV infection using pre- and post-contrast FLAIR imaging and to develop a toolbox approach to assessing meningeal inflammation in a variety of other infectious disease entities.Methods
Six cynomolgus macaques (3-6 kg) that had been pre-screened for LASV antibodies were inoculated to 1000 PFU LASV in the right triceps. All animals underwent 60 minutes of imaging on a Philips Achieva 3 Tesla clinical MR scanner (Philips Healthcare, Cleveland, OH, USA), using an 8-channel pediatric neuro-spine coil. Following anesthesia, subjects were intubated, and gadobutrol contrast agent (0.1ml/kg, Bayer Inc. Albany, NY, USA) was used for post-contrast imaging. Among other sequences, axial pre- and post-contrast FLAIR and T1-weighted (T1w) images were obtained at two baselines (BL) and immediately prior to euthanasia (Terminal Day, DT). One animal (subject 1) could not be scanned prior to euthanasia due to rapid clinical decline. Percent change maps were created from pre- and post-contrast FLAIR and pre- and post-contrast T1w images using a MIM extension (MIM Software v. 6.9, Cleveland Ohio) (Figure 1A-C). The D99 rhesus macaque digital brain atlas3 was used to create the volumes of interests (VOIs) on the synthetic T1w image (Figure 1D). A co-registered T1w brain scan was automatically de-skulled and segmented into gray matter, white matter and cerebrospinal fluid (CSF). A MIM workflow (MIM Software v. 6.9, Cleveland Ohio) was then used to create five VOIs: total white matter, cortical gray matter, inner CSF (primarily ventricles), outer CSF (includes subarachnoid space and meninges) and deep gray matter (thalamus, caudate and putamen, located using Figure 1D). The percent change values of each VOI on FLAIR and T1w % change maps were measured and graphed to compare difference between averaged BL and DT. Laboratory assays and physical examinations were performed throughout the disease process. Necropsy was performed with gross pathologic and histologic correlations. Images and VOIs were reviewed by a neuroradiologist.Results
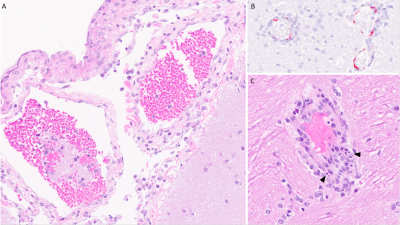
Pathologic meningeal enhancement following LASV exposure was detected more clearly on FLAIR images compared to T1w images. Although both sequences showed increased enhancement on DT, the magnitude of change was much more appreciable on FLAIR images compared to T1 weighted images (Figures 2, 3 and 4). Those changes on post-contrast FLAIR were most noticeable in the outer CSF (meningeal), inner CSF (ventricles) and cortical gray matter (Figure 3, 4). Histopathology confirmed viral meningoencephalitis (Figure 5).Discussion
Viral meningitis was assessed quantitatively in five animals infected with LASV, but to different extents using subtracted pre- and post-contrast FLAIR images. The increase in enhancement in the outer CSF likely reflects a combination of meningeal enhancement and contrast leakage. Increased enhancement in the cortical gray matter probably reflects inflammatory changes within the cortex related to adjacent meningeal inflammation. Increased enhancement of the inner CSF encompasses choroid plexus enhancement along with contrast leakage due to meningeal inflammation. Enhanced FLAIR images better assessed the degree of meningeal enhancement compared to T1w images. The subject (S4) with the largest increase in meningeal enhancement on FLAIR (75%) was the most symptomatic with tremors noted on terminal day. The viral load, and immunology data will be analyzed and compared with imaging data.Conclusion
We quantitatively assessed the degree of meningeal inflammation in an animal model of LASV infection in a high containment setting using subtraction of pre- and post-contrast FLAIR images. This approach was more sensitive than subtracting pre- and post-contrast T1w images and seemed to correlate with the severity of disease. This technique can be used in other infectious diseases where subtle or appreciable meningitis is suspected of playing a role in the pathophysiology of disease. Understanding the CNS manifestations of LASV infection will then provide a solid background for evaluation of vaccine and therapeutic studies in the future.Acknowledgements
Animal Ethics Statement: Animals were housed in an AAALAC-International-accredited facility. All experimental procedures were approved by the NIAID Division of Clinical Research (DCR) Animal Care and Use Committee and were in compliance with the Animal Welfare Act regulations, Public Health Service policy, and the Guide for the Care and Use of Laboratory Animals recommendations.
Funding: This work was supported by NIAID Division of Intramural Research and NIAID DCR and was performed under Battelle Memorial Institute contract (No. HHSN272200700016I) with NIAID. Additional support was provided by the NCI Contract No. HHSN261200800001E.
Acknowledgements: The authors appreciate Division of Microbiology and Infectious Diseases for providing animals. We would also like to thank the Comparative Medicine, Clinical Services, Histology and Imaging teams at the Integrated Research Facility (IRF) without whose effort this work could not be completed.
References
1. Okokhere, Peter O et al. Aseptic meningitis caused by Lassa virus: Case series report. Case Reports Neurol Med. 2016;2016:1978461. doi:10.1155/2016/1978461
2. Mateer, Elizabeth J et al. Lassa fever-induced sensorineural hearing loss: A neglected public health and social burden. PLoS Negl Tropl Dis. 2018;12(2):e0006187. doi:10.1371/journal.pntd.0006187
3. Reveley, Colin et al. Three-dimensional digital template atlas of the macaque brain. Cereb Cortex. 2017;27(9):4463-4477. doi:10.1093/cercor/bhw248
Figures