2023
Diagnostic utility of Geometric Solution bSSFP in the internal auditory canal and orbits1Radiology, University of Washington, Seattle, WA, United States, 2Radiology, University of Washington, SEATTLE, WA, United States
Synopsis
Imaging pathology in the internal auditory canal and orbits benefits from techniques such as bSSFP MRI due to its high time efficiency and SNR. Here the geometric solution (GS) is proposed in concert with bSSFP for temporal bone and orbit imaging to circumvent banding and motion artifacts. The GS-bSSFP technique is validated in a cohort of patients scheduled for evaluation of treated vestibular schwannoma. Three blinded neuroradiologists found that GS-bSSFP exceeded comparable techniques in terms of image quality and diagnostic utility.
Introduction
High signal, resolution, and fluid/tissue contrast are advantageous for discrimination of pathology in the internal auditory canal (IAC) and orbits. Congenital ear anomalies, vestibular schwannoma, inner/middle ear lesions, retinal/choroidal detachment, and intraocular tumors including lymphoma, melanoma, and ocular surface squamous neoplasia have an increased probability of detection when image quality is high.Balanced steady state free precession (bSSFP) has excellent SNR efficiency and T1/T2 ratio contrast that reliably differentiates solid from aqueous tissue, but suffers from artifacts such as dark banding and motion ghosting/blur/dephasing in the IAC and orbits. These artifacts can originate from variable tissue susceptibility, aqueous/vitreous humor flow, ocular motion, CSF flow, and cisternal vessel pulsation artifact. Luckily, a geometric solution (GS) of phase-cycled bSSFP MRI has been developed that corrects most of these artifacts1,2 while maintaining high contrast and SNR. Here the GS is validated for clinical efficacy in the IAC and orbits relative to standard bSSFP and a complex sum (CS) of phase-cycled bSSFP.
Methods
An institutional review board approved this study that involved 21 patients undergoing a clinically indicated temporal bone (IAC protocol) MRI for evaluation of treated vestibular schwannoma. The patients received added bSSFP MRI sequences acquired with 0°, 90°, 180°, and 270° phase cycling in both sagittal and axial orientations on Philips Ingenia 3T scanners. Scan parameters were FA/TR/TE = 30°-45°/4.8-5.5ms/1.9-2.2ms, 316/314-316/42-100 matrix size and 0.57/0.57/1mm voxel size along frequency/phase/slice directions, respectively.The GS and CS were computed on the same four phase-cycled bSSFP MRI datasets as previously described1,3, and signal-normalized to avoid bias. Three fellowship-trained neuroradiologists blinded to sequence type, imaging parameters, clinical presentation, and patient disposition evaluated standard bSSFP, GS-bSSFP, and CS-bSSFP for assessment of image quality (using an ordinal 5-point Likert scale with 1 = low to 5 = high quality) and diagnostic utility (readers were asked to choose which technique(s) had the best diagnostic utility). Image quality ratings were compared between techniques using the Wilcoxon rank-sum test or the Sign test, clustered by subject to account for multiple readers.
Results
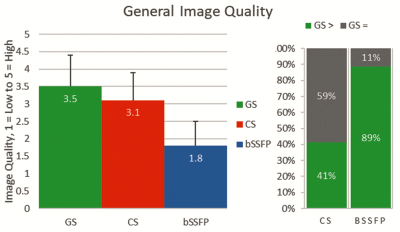
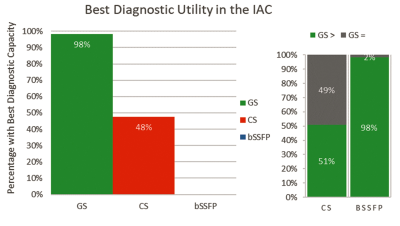
Figure 1 illustrates that the GS was deemed to have greater image quality than both the CS and standard bSSFP (p<0.001 for both). Image quality of the GS was rated better than the CS in 41% and the same in 59% of reads, and better than bSSFP in 89% and the same in 11% of reads; the GS was never rated worse than the CS or standard bSSFP.Figure 2 shows that the GS was deemed to have greater diagnostic utility in the IAC than the CS and standard bSSFP (98% vs. 48% and 98% vs. 0% respectively, p<0.001 for both). GS was rated as being as least as good as the CS and bSSFP in all reads, better than CS in 51%, and better than bSSFP in 98%.
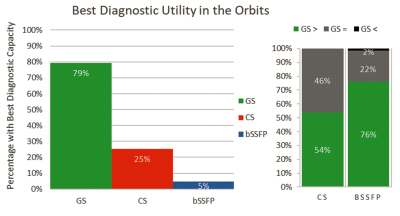
Figure 3 shows that the GS was assessed to have greater diagnostic utility in the orbits than the CS and standard bSSFP (79% vs. 25% and 79% vs. 5% respectively, p<0.001 for both). While the GS was never deemed to have less diagnostic utility in the orbits than the CS, 1 of 63 (~2%) reads recorded the GS to have less diagnostic utility than bSSFP. Otherwise, GS was rated as being better than CS in 54%, and better than bSSFP in 76% of orbit reads.
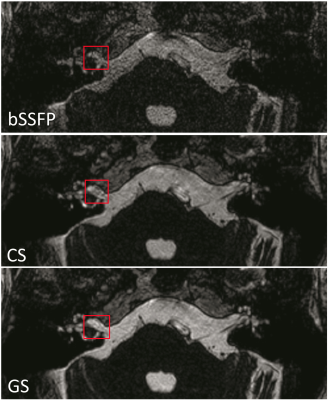
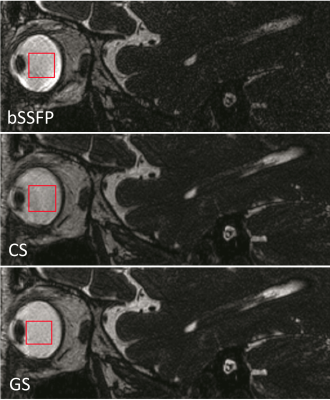
Figure 4 depicts the three techniques imaging an IAC, where there are subtle artifacts anterior to the right IAC lesion that are less noticeable in the GS. Figure 5 shows the three techniques imaging the orbits, where the globe contours are smooth and well-demarcated with homogeneously bright vitreous humor in the GS relative to the CS and standard bSSFP.
Discussion
GS-bSSFP in the IAC and orbits has shown to provide a robust aid to pathological diagnosis. Although readers often disagreed on the ratings of individual cases, GS quality and utility was never gauged inferior to the CS. The same applies to comparisons with bSSFP, except for one read amongst 3 readers x 21 patients x 3 metrics = 189 assessments. This would appear to be an anomaly; standard bSSFP should always be inferior to the GS and CS, since they are both effectively weighted averages of four phase-cycled bSSFP images and should thus have greater SNR than each standalone bSSFP image.Conclusion
The use of the GS in conjuncture with bSSFP imaging has proven to be a powerful tool for neurological diagnosis in the IAC and orbits, and was never found to be inferior to the current implementation of the CS.Acknowledgements
NoneReferences
[1] Xiang QS, Hoff MN. Banding artifact removal for bSSFP imaging with an elliptical signal model. Magn Reson Med. 2014;71:927–933.
[2] Hoff MN, Andre JB, Xiang QS. On the Resilience of GS-bSSFP to Motion and other Noise-like Artifacts, Proc ISMRM 2015; 23:818.
[3] Hoff MN, Andre JB, Xiang QS. Combined Geometric and Algebraic Solutions for Removal of bSSFP Banding Artifacts with Performance Comparisons, Magn Reson Med. 2017;77:644–654.
Figures