2017
Brain activation patterns associated with sexual orientation and its correlation with free testosterone in female-to-male transsexuals1Advanced Institute of Aging Science, Chonnam National University, Gwangju, Korea, Republic of, 2Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States, 3Department of Urology, Chonnam National University Hospital, Gwangju, Korea, Republic of, 4Department of Radiology, Chonnam National University Medical School, Gwangju, Korea, Republic of
Synopsis
Recent functional magnetic resonance imaging studies revealed that the sexual orientation of male-to-female transsexuals have a specific tendency of female-like brain function during sexual arousal; however, the relationships between brain morphology and brain activation patterns reflecting sexual orientation in postoperative female-to-male (FTM) transsexuals are currently unknown. The purpose of this study was to evaluate the brain activation pattern associated with sexual orientation and its correlation with the level of the free testosterone in postoperative FTM transsexuals. This study was also performed to discriminate the gray and white matter volume differences between FTM transsexuals and female controls.
Introduction
Transsexualism is a gender identity disorder characterized by a stated desire to be the opposite sex and a persistent discomfort with one’s natal body, compelling individuals to undergo cross-sex hormone treatment and surgical therapy aimed at making their body as congruent as possible with the opposite sex (1). Transsexualism is a rare condition affecting approximately one in 12,000 males and one in 30,000 females (2). The FTM transsexuals have male genitalia by a sex reassignment surgery, and can experience sexual arousal and attraction to females as the same genetic sex. Sexual hormones contribute differently to the gender-brain development interaction, with specific areas that seem to respond to them specifically (1,3). Especially, testosterone is essential for the development of the male reproductive system, and plays an important role in the regulation of the central nervous system function. Testosterone administration in FTM transsexuals had a positive effect on sexual function and mood (4). Therefore, the effect of testosterone treatment in FTM transsexuals leads to increased sexual arousal. We assumed that morphometric and functional changes are closely related to each other, and the combined results provide a clue to gain an important understanding of the sexual orientation in FTM transsexuals.Methods
Eleven postoperative FTM transsexuals (mean age: 41.5±5.5 years) with sex reassignment surgery and 16 female controls (mean age: 43.7 ± 5.0 years) participated in this study. The postoperative FTM transsexuals underwent a sex reassignment surgery to alter their bodies at the Dong-A University Hospital, and have been treated with hormone supplementary therapy. Eleven FTM transsexuals underwent fMRI on a 3.0T MR scanner. The visual sexual stimulation began with a thirty second neutral pictures, 60 second stimulation with a string of female nude pictures, 30 second neutral pictures, 60 second stimulation with a string of male nude pictures, 30 second neutral pictures, 60 second stimulation with a string of male and female nude pictures and concluded with 30 second neutral pictures. MRI and fMRI data were post-processed using Statistical Parametric Mapping (SPM8) software with the diffeomorphic anatomical registration through an exponentiated Lie algebra (DARTEL) algorithm.Results and discussion
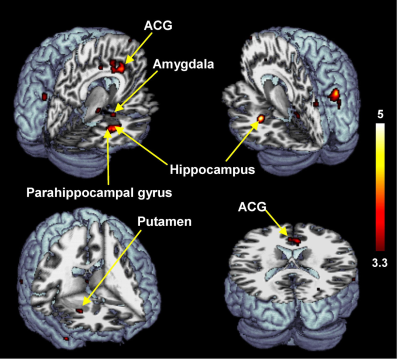
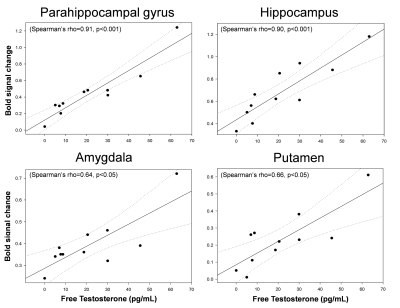
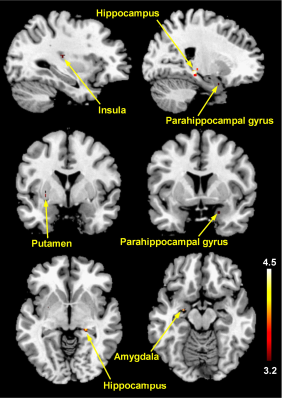
The reported scores for perceived sexual arousal in FTM transsexuals were 2.5 ± 1.1 and 1.2 ± 0.9 in females and males nude pictures, respectively (p=0.009). The average levels of the free-T, sex hormone binding globulin, estradiol, follicle stimulating hormone (FSH), and luteinizing hormone in FTM transsexuals were 21.5±19.3 pg/ml, 32.0±14.5 nmol/L, 48.4±33.9 pg/ml, 28.7±32.5 mlU/ml, and 20.7±23.0 mlU/ml, respectively. The level of the FSH was in the average range of the postmenopausal women, whereas the rest of the sexual hormones were in the average range of the normal men.The brain areas with predominant activities in FTM transsexuals during viewing female erotic nude pictures as contrast to male nude pictures included the hippocampus, parahippocampal gyrus, anterior cingulate gyrus (ACG), amygdala, and putamen (p<0.005) (Figure 1). The distinct brain areas with predominant activities were not found during viewing male nude pictures over female pictures. It is noteworthy that the free-T levels were positively correlated with BOLD signal changes in the hippocampus (Spearman’s rho=0.90, p<0.001), parahippocampal gyrus (rho=0.91, p<0.001), amygdala (rho=0.64, p<0.05), and putamen (rho=0.66, p<0.05) (Figure 2). The eleven FTM transsexuals were divided into two groups based on the free-T levels as follows: Group I (n=7) consisting of 7 subjects whose average level of the free-T was 25.6±18.8 pg/ml that is in the normal range of men and Group II (n=4) consisting of 4 subjects whose average level was 4.6±3.1 pg/ml that falls in to the deficient range. The predominant activation areas observed in Group I as contrast to Group II included the hippocampus, amygdala, putamen, and parahippocampal gyrus during viewing the female nude pictures (Figure 3).
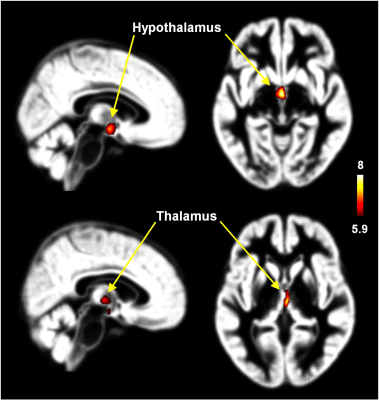
The FTM transsexuals showed significantly (p < 0.05) larger GM volumes in the thalamus and hypothalamus compared with female controls (Figure 4). However, there were no brain areas with larger WM volume in female controls compared with FTM transsexuals. The GM volumes of the thalamus and hypothalamus in the postoperative MTF transsexuals in our study are not directly correlated with brain activity in the hippocampus, parahippocampal gyrus, ACG, amygdala, and putamen when viewing erotic female nude pictures.
Conclusion
This study revealed the specific brain activation pattern associated with sexual orientation and its correlation with the free-T in the postoperative FTM transsexuals. These findings would be helpful to understand the neural mechanisms on sexual arousal in connection with sexual hormone level in the FTM transsexuals.Acknowledgements
This research was supported by the grants from NationalResearch Foundation funded by the Korea government(Ministry of Science and Information and Communication Technology; 2018R1A2B2006260 and 2018R1C1B6005456), and by the Chonnam National University (CNU)Research Fund for CNU distinguished research professor (2017-2022).References
1. Santarnecchi E, Vatti G, Dettore D, Rossi A (2012) Intrinsic cerebral connectivity analysis in an untreated female-to-male transsexual subject: a first attempt using resting-state fMRI. Neuroendocrinology 96:188-193.
2. Meriggiola MC, Berra M (2012) Long-term cross-sex hormone treatment is safe in transsexual subjects. Asian J Androl 14:813-814.
3. Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF (2000) Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425:422-4354.
4. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC (2013) A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther 39:321-335.
Figures