2016
Real-time phase contrast MRI measurements of coughing in patients with Chiari malformation1Neuroscience Research Australia, Randwick, Australia, 2Prince of Wales Clinical School, University of New South Wales, Randwick, Australia, 3Philips Australia & New Zealand, North Ryde, Australia, 4Department of Clinical Medicine, Macquarie University, Macquarie Park, Australia
Synopsis
Cerebrospinal fluid (CSF) flow, as characterised by real-time PCMRI, may inform surgeons whether a patient with Chiari malformation may benefit from decompression surgery. 5 healthy controls and 7 Chiari patients with (N=4) and without (N=3) coughing headache underwent MRI scanning to measure CSF velocities while coughing. Results suggest that Chiari patients with coughing triggered headaches have increased CSF velocities at the foramen magnum during a cough compared with both controls and patients without headache. This may help understand the mechanisms of coughing triggered headaches and provide a useful indicator for clinicians.
Introduction
Chiari malformation is a congenital disorder where the cerebellar tonsils herniate through the foramen magnum into the upper spinal canal1, however a link between the anatomy and the symptoms, such as coughing associated headaches, has yet to be demonstrated. The current treatments for Chiari are not evidenced based, and since the mechanisms for coughing associated headache are unknown, there are no standardised indications for when surgical intervention is required, or how patients should be treated. This leads to variable, and often unsatisfactory outcomes2, 3.Invasive pressure measurements have been used to examine the association between Chiari and coughing triggered headache. From these studies it has been assumed that the cerebellar tonsils obstruct normal flow through the foramen magnum, creating pressure gradients between the cranium and spinal canal that may contribute to headaches4-6. Recently, pencil beam MRI has been used to demonstrate the amplitude of cerebrospinal fluid (CSF) pulsation was reduced post-cough7, 8, suggesting this may be an objective measure of a cough induced obstruction. However, pencil beam MRI cannot characterise important local differences in CSF flow. The aim of this study was to use 2D real-time phase contrast MRI (PCMRI) to determine whether the cerebellar tonsils restrict CSF flow while coughing, and to develop an imaging based indicator for surgical intervention.
Methods
Chiari malformation patients with (N = 4) and without (N = 3) coughing associated headaches (females aged 24-45 years), and 5 healthy controls (females aged 27-50 years) were scanned in a clinical 3T MRI scanner (Achieva TX, Philips Healthcare, Best, The Netherlands), using a 16-channel neurovascular coil.During scanning, participants were instructed to cough between normal breaths. To measure the effects of coughing on CSF flow real-time PCMRI was used. The imaging planes were positioned at the foramen magnum and at the middle of the C3 vertebra, perpendicular to the direction of flow. 200 phases were acquired at 70 ms intervals, with encoding velocities between 10-40 cm.s-1. Imaging parameters include: TR/TE = 13/7 ms, FOV = 192×192mm, slice thickness = 10 mm, in-plane resolution = 1.5 mm. Regions of interest for the CSF spaces were drawn manually, and the CSF velocities were calculated using Segment9. The rostral and caudal amplitude of the CSF pulse during a cough, and the cardiac pulses post-cough were recorded for comparison between groups.
Results
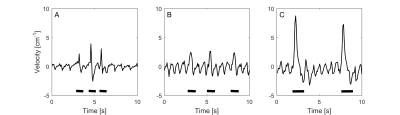
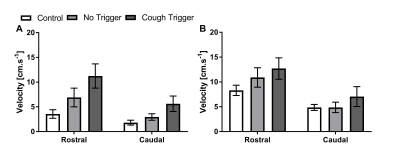
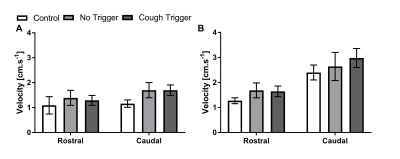
Figure 1 shows typical CSF velocity-time data at the foramen magnum while coughing. In all subjects, during the cough there was an initial increase in rostral flow, followed by a caudal return. After the cough, cardiac pulsation quickly returned. Figures 2 and 3 show the average peak velocities at both locations during and after a cough respectively. During a cough the CSF flow velocities at the foramen magnum in patients with coughing headaches (rostral: 11.2±2.5 cm.s-1, caudal: 5.6±1.7 cm.s-1) tended to be greater than patients without (rostral: 6.9±1.9 cm.s-1, caudal: 3.0±0.7 cm.s-1) and controls (rostral: 3.6±0.9 cm.s-1, caudal: 1.8±0.5 cm.s-1) (Figure 2A). Post-cough, the amplitude of the cardiac pulsations were similar between all groups, at both the foramen magnum and C3 (Figure 3).Conclusion
These results suggest that coughing does not cause the tonsils to restrict flow. Rather, the tonsillar obstruction may create high velocity CSF flow at the foramen magnum, which could contribute to the symptoms of coughing associated headache in Chiari patients. This characteristic flow may provide a useful indicator as to which patients would benefit the most from posterior decompression surgery. These results need confirmation in a larger population.Acknowledgements
This study was funded by the Column of Hope. L.E.B. is supported by an Australian National Health and Medical Research Council (NHMRC) senior research fellowship. I.K.B. is employed by Philips Australia & New Zealand.References
1. Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44.
2. Zhao JL, Li MH, Wang CL, Meng W. A Systematic Review of Chiari I Malformation: Techniques and Outcomes. World Neurosurg. 2016;88:7-14.
3. Jia C, Li H, Wu J, Gao K, Zhao CB, Li M, et al. Comparison decompression by duraplasty or cerebellar tonsillectomy for Chiari malformation-I complicated with syringomyelia. Clin Neurol Neurosurg. 2019;176:1-7.
4. Williams B. Simultaneous cerebral and spinal fluid pressure recordings. II. Cerebrospinal dissociation with lesions at the foramen magnum. Acta Neurochir. 1981;59(1):123-42.
5. Tachibana S, Iida H, Yada K. Significance of positive Queckenstedt test in patients with syringomyelia associated with Arnold-Chiari malformations. J Neurosurg. 1992;76(1):67-71.
6. Heiss JD, Snyder K, Peterson MM, Patronas NJ, Butman JA, Smith RK, et al. Pathophysiology of primary spinal syringomyelia. J Neurosurg Spine. 2012;17(5):367-80.
7. Bezuidenhout AF, Khatami D, Heilman CB, Kasper EM, Patz S, Madan N, et al. Relationship between Cough-Associated Changes in CSF Flow and Disease Severity in Chiari I Malformation: An Exploratory Study Using Real-Time MRI. AJNR. 2018;39(7):1267.
8. Bhadelia RA, Patz S, Heilman C, Khatami D, Kasper E, Zhao Y, et al. Cough-Associated Changes in CSF Flow in Chiari I Malformation Evaluated by Real-Time MRI. AJNR. 2016;37(5):825-30.
9. Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1.
Figures