2011
An MRI traumatic brain injury case study at 7 Tesla: pre- and post-injury structural network and volumetric reorganization and recovery1Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom, 2Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai / University of Cambridge, New York, NY, United States, 3Brain Injury Research Centre, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5Department of Computer Science, Mathematics, Physics, and Statistics, University of British Columbia, Kelowna, BC, Canada
Synopsis
This case study investigates the structural effects of traumatic brain injury for the first time using pre-injury and post-injury 7 Tesla MRI longitudinal data. We report findings of initial volumetric changes, decreased structural connectivity and reduced microstructural order that appear to return to baseline 8 months post-injury, suggestive of long-term plasticity and recovery.
Methods: An adult female patient was knocked over by a car, causing a loss of consciousness for several seconds. The post-injury CT scan showed subcutaneous soft tissue swelling over the right parietal bone. The patient had undergone two scanning sessions at 7 Tesla prior to the head injury as a healthy control research participant. Two more scans were acquired post-injury. The scan dates were as follows: February 2016, January 2017, January 2018 and August 2018. Included in each protocol was a T1-weighted Magnetization Prepared 2 Rapid Acquisition Gradient Echo Echo (MP2RAGE) (1), a T2-weighted Turbo Spin Echo (TSE), and a diffusion MRI (dMRI). The MP2RAGE high spatial resolution (1) voxel size was 0.8 mm isotropic, TR/TE = 6000/3.2 ms and TI1(θ1)/TI2(θ2) = 1050(5⁰)/3000(4⁰) ms. Two TSE structural images were obtained at high in-plane resolution (0.4 x 0.4 mm2) and a slice thickness of 2 mm. TR/TE = 6900/69 ms, and θ = 150⁰. The first T2-TSE was obtained in a coronal-oblique orientation where the imaging plane was aligned perpendicular to the long axis of the hippocampus. The second T2-TSE was obtained in an axial orientation, the imaging plane aligned along the axis connecting the anterior commissure and the posterior commissure (AC-PC). Diffusion MRI were also acquired at each scan timepoint, using a single-shot spin-EPI sequence aligned axially with an isotropic resolution of 1.05 mm and TR/TE = 6900/67 ms. The diffusion sequence was a paired acquisition with reversed phase encoding in the AP/PA direction. Each pair had 64 diffusion encoding directions (b=1200 s/mm2) and 4 unweighted scans (b=0 s/mm2). The FreeSurfer ‘recon-all’ pipeline (version 6.0) (2) was used to process the T1-weighted structural data at submillimetre resolution (3). Hippocampal subfield (4) and amygdala subnuclei segmentation (5) were also carried out using FreeSurfer. A multispectral segmentation approach was used, utilizing both the T1-weighted and T2-weighted images, leveraging the enhanced resolution of the T2-weighted image to provide additional anatomical information. Structural connectomes at different timepoints were compared by custom functions that performed elementwise subtraction of the matrices in MATLAB. To determine a streamline threshold of the connectome with an acceptable level of variability, mean matrix co-efficients of variation were calculated for the following streamline thresholds: 25, 50, 100, 200, 400, 800, 1600, 3200, 6400, 12800 and 25600 (Fig. 1). Actual streamline thresholding was set at 15000, discarding edges consisting of streamline bundles with less density than the threshold. Volumetrics, fractional anisotropy (FA) and connectivity were analysed at each of the 4 time points and compared.
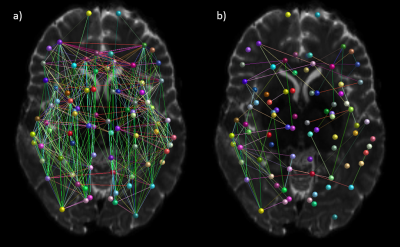
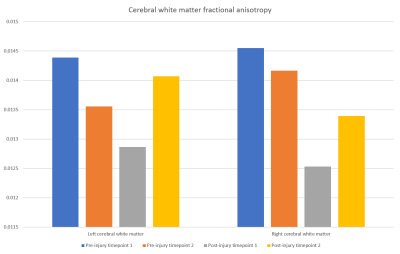
Results: The subcortical segmentation of the amygdala nuclei and hippocampal subfields did not reveal any clear changes between the scanning timepoints (Fig. 2). At post-injury timepoint 1, the right and left hemispheric brain segmentation revealed lower cortical grey matter and cerebral white matter volume compared to other scanning timepoints. No change was apparent in ventricle volume (Fig. 3). Mean post-injury structural connectivity displayed widespread reduction in the network connection density compared to mean pre-injury data. A comparison between the mean pre-injury connectome and the two post-injury matrices was then carried out, to investigate if changes to the patient’s structural connectivity post-TBI was consistent over time. The results showed that at post-injury timepoint 1, connection density was extensively reduced, but this decrease in connectivity was much diminished by post-injury timepoint 2 (Fig. 4). Concurrent with the changes in the structural connectome and volumetrics, fractional anisotropy (FA) of the cerebral white matter was markedly reduced in both hemispheres in the first scan following the head trauma. In both the left and right hemispheres, the final timepoint scan revealed a subsequent increase of FA to levels similar to those pre-injury (Fig. 5).
Discussion: Here, our case data show that the post-traumatic injury brain exhibits widespread alterations to the cortical grey matter and cerebral white matter volume. Additionally, white matter connectivity displays generalized decreased connection strength across the network, both in terms of connection density and microstructural order of the tissue. Interestingly, structural changes attributed to head trauma are no longer evident by the final scanning timepoint, suggestive of long-term plasticity and recovery.
Conclusion: This case study investigates the structural effects of traumatic brain injury for the first time using pre-injury and post-injury 7 Tesla MRI longitudinal data. We report findings of initial volumetric changes, decreased structural connectivity and reduced microstructural order that appear to return to baseline 8 months post-injury.
Acknowledgements
No acknowledgement found.References
1. Marques JP, Gruetter R. New developments and applications
of the MP2RAGE sequence--focusing the contrast and high spatial resolution R1
mapping. PLoS One 2013;8(7):e69294. doi: 10.1371/journal.pone.0069294
2. Fischl B, Salat DH, Busa E, Albert M, Dieterich M,
Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A,
Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of
neuroanatomical structures in the human brain. Neuron 2002;33(3):341-355.
3. Zaretskaya N, Fischl B, Reuter M, Renvall V, Polimeni JR.
Advantages of cortical surface reconstruction using submillimeter 7 T MEMPRAGE.
Neuroimage 2018;165:11-26. doi: 10.1016/j.neuroimage.2017.09.060
4. Iglesias JE, Augustinack, J.C., Nguyen, K., Player, C.M.,
Player, A., Wright, M., Roy, N., Frosch, M.P., Mc Kee, A.C., Wald, L.L.,
Fischl, B., and Van Leemput, K. A computational atlas of the hippocampal
formation using ex vivo, ultra-high resolution MRI: Application to adaptive
segmentation of in vivo MRI. Neuroimage 2015;115(117-137).
5. Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW,
Boyd E, Reuter M, Stevens A, Van Leemput K, McKee A, Frosch MP, Fischl B,
Augustinack JC, Alzheimer's Disease Neuroimaging I. High-resolution magnetic
resonance imaging reveals nuclei of the human amygdala: manual segmentation to
automatic atlas. Neuroimage 2017;155:370-382. doi:
10.1016/j.neuroimage.2017.04.046
Figures
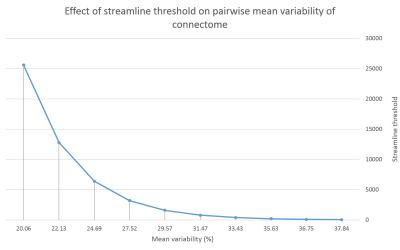
Fig. 1. Streamline threshold effect on variability of the connectome: averaged element-wise change of connectivity matrix between pre-injury timepoint 1 and pre-injury timepoint 2.

Fig. 2. a) Hippocampal subfield segmentation as performed by FreeSurfer, into dentate gyrus, subicular complex, CA1 and CA3/4 and b) amygdala subnuclei segmentation as performed by FreeSurfer into the basal, lateral, accessory basal, central, cortical and medial nuclei.
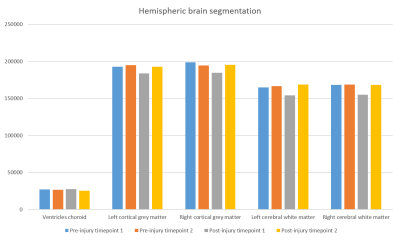
Fig. 3. Ventricle volume and hemispheric volumes of the cortical grey matter and cerebral white matter at each scanning timepoint.