2010
Evolution of Cerebral Hemodynamics with Migraine Onset and Propagation: a 21.1 T Study
Nastaren Abad1,2, Hannah Elizabeth Alderson1,2, Michael Gordon Harrington3, and Samuel Colles Grant1,2
1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States, 3Neurosciences, Huntington Medical Research Institutes, Pasadena, CA, United States
1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States, 3Neurosciences, Huntington Medical Research Institutes, Pasadena, CA, United States
Synopsis
Dysfunctional ionic and metabolic regulation is evident in migraine, preceeding behavioral onset of pain and culminating in the extensive clinical (pre, peri and post-ictal) features presented in migraine. It, therefore, stands to reason that the ionic and metabolic abnormalities may induce microvascular blood flow changes in brain tissue, with altered perfusion not as an inciting event but as a response underlying ionic impacts. To elucidate the timing of hemodynamic effects in migraine, the overarching aim of this study is to investigate perfusion changes that potentially follow triggered migraine.
Introduction
Migraine is a recurrent disabling neurological disorder, characterization of which is confounded by multiple triggers and physiological repercussions. Afflicting more than 38 million in the USA, migraines render sufferers unable to perform everyday activities and affecting quality of life, which has led the World Health Organization to identify migraine as the 5th most disabling disorder worldwide1. A fundamental understanding of migraine progression is lacking, and current therapeutic intervention are largely palliative. Previous work has established a link between dysfunctional regulation of sodium and altered metabolism with migraine onset and progression2,3. While differential impacts on blood flow are anticipated particularly with the establishment of sodium propagated hyper-excitability, associated perfusion changes may be a functional response to the propagation. To elucidate the timing of hemodynamic effects in migraine, the overarching aim of this study was to investigate perfusion changes that potentially follow nitroglycerin (NTG)-triggered migraine. To track these functional changes, neuroanatomically specific cerebral blood flow (CBF) measurements in triggered migraine versus control were mapped longitudinally.Materials & Methods
Animal Model: Using an acute preclinical analogue of migraine based on NTG injection, twelve Sprague-Dawley male rats were imaged. While anesthetized in the MR scanner, the rats (n=6/group) were administered in situ an IP injection of either NTG to trigger the migraine or saline to serve as control.MR Acquisitions: Using the 21.1-T ultra-widebore magnet at the US National High Magnetic Field Laboratory and a linear 1H coil. To track evolving perfusion with potentially rapid onset during central sensitization, an Echo Planar Imaging (EPI) ASL implementation (EPI-ASL) was utilized to allow for fast acquisition times. Slice-selective and non-selective inversions were acquired using an interleaved acquisition with a TR of 6.5 s to allow the magnetization to return to equilibrium between repetitions and limit the duty cycle of the magnetic field gradients. An 11-ms hyperbolic secant was used to provide a uniform inversion profile for both selective and non-selective inversion, with inversion delays ranging from 50-3000 ms. With partial Fourier encoding and four segments (yielding a minimum TE = 8.8 ms) , the total scan time was 7 min to acquire five slices. Over a FOV of 3.0 x 3.0 cm, the imaging matrix for perfusion images and T1 maps was 128 x 128 with a through-plane resolution of 1.0 mm. A pre-injection baseline was established by repeatedly scanning for 30-min pre-injection. Post NTG injection, ASL scans were acquired for >2 h to develop a novel time course of hemodynamic evolution over migraine onset and propagation.
Data Analysis: To calculate CBF, a custom script was implemented in MATLAB® for a pixelwise fit to obtain both specific and non-specific T1 maps4. A constant tissue/blood partition coefficient (𝜆) of 0.9 mL/g was used. A region of interest (ROI) analysis was used to calculate mean perfusion over the thalamus, neocortex and brainstem. Flow-weighted images and CBF maps were obtained, and data was quantified as percent change from baseline (pre-injection). All datasets were analyzed statistically using JMP PRO 14.0 (SAS Institute, Cary, NC). For ROI analysis, relative CBF values (mean ± SD) at each time point were compared between groups (NTG and saline) using a Student's t-test (*p<0.05) as well as a comparison to baseline (†p<0.05).
Results
Representative perfusion-weighted images and CBF maps are demonstrated for two coronal slices in the rodent brain (Figure 1), delineating good contrast in signal between gray and white matter. With a total acquisition time of 7 min, cerebral blood flow values in naive rodent brains range between 20-300 mL/min/g of tissue. Uniquely, with NTG trigger, hyper-perfusion is evident in both brainstem and thalamus, albeit transiently, but with a similar time course (Figure 2). Microperfusion alterations in brainstem demonstrated transient elevations gaining significance compared to both baseline and control, with maximal percent change in perfusion at 48 ± 20 %. Notably, the neocortex did not demonstrate any significance with baseline or control for the entire duration of the time course investigated.Discussion & Conclusion
Overall, the present study demonstrates differential cerebral microcirculation, with differential alterations in trigeminovascular system and cortical structures highlighting the complex interplay implicit in migraine pathophysiology. Altered perfusion significantly impacting the brainstem with elevations in the thalamus, both of which are gateways to integration of nociceptive inputs and the pain matrix. However, CBF increases are largely subsequent to both ionic and metabolic changes that have been established in migraine progression2,3. Given the transient nature of perfusion changes and the preceding and lasting impacts on ionic and metabolic factors, perfusion impacts appear to be a functional response to the instituted hyper-excitability rather than a causative mechanism.Acknowledgements
This work was supported by the NIH (RO1-NS102395) and User Collaborations Grant Program (to SCG) from the National High Magnetic Field Laboratory, which is funded by the NSF (DMR-1644779), the NHMFL REU program, and the State of Florida.References
- Bes A, et al. The international classification of headache disorders, 3rd ed. Cephalalgia. 2013; 33(9): 629-808.
- Abad N, Rosenberg JT, Hike DC, Harrington MG, Grant SC. Dynamic sodium imaging at ultra-high field reveals progression in a preclinical migraine model. Pain. 2018;159(10): 2058-65.
- Abad N, Rosenberg JT, Roussel T, Grice DC, Harrington MG, Grant SC. Metabolic assessment of a migraine model using relaxation-enhanced 1H spectroscopy at ultrahigh field. Magn Reson Med. 2017; 79: 1266-1275.
- Kwong K, Chesler D, Weisskoff R, Donahue K, Davis T, Ostergaard L, Campbell T, Rosen B. MR perfusion studies with T1-weighted echo-planar imaging. Magn Reson Med. 1995; 34(6): 878-87.
Figures
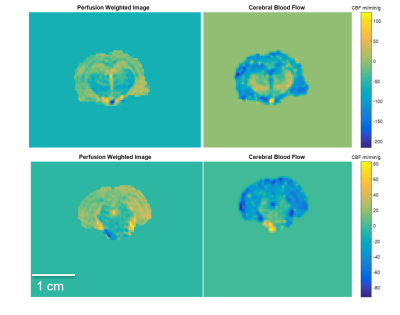
Figure 1. Representative images from coronal slices in the rodent brain indicating perfusion weighted (left column) and cerebral blood flow (right column) maps for slices including the thalamus (top) and brainstem (bottom).
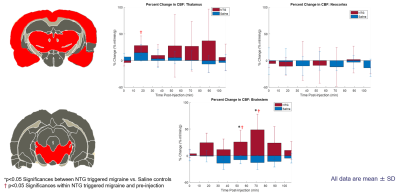
Figure 2. Representative images from coronal slices in the rodent brain indicating regions of interest segmented using a rodent atlas corresponding to the thalamus, neocortex and brainstem. Bar plots of percent change in CBF between NTG and saline rodents are displayed for these ROI as a function of time post injection. Percent change is reported as mean CBF (mean % change ± SD).