2008
Clinical validation of patient-specific parcellation of the STN using 7T MRI via a DBS electrode placement revision case1University of Minnesota, Minneapolis, MN, United States
Synopsis
Deep brain stimulation (DBS) in the subthalamic nucleus (STN) is an effective therapy for the motor signs associated with Parkinson’s disease. The STN is organized into three main functional territories, motor, associative and limbic, that can be identified using structural connectivity-based parcellation. While many may argue that the DBS electrode should be implanted in the sensorimotor region for maximum motor benefits, the optimal location within the STN remains under debate. In this study we describe a patient who experienced STN-DBS-induced depression, which was significantly alleviated following revision of the electrode. A 7T imaging-based reconstruction provides an explanation for this observation.
Introduction
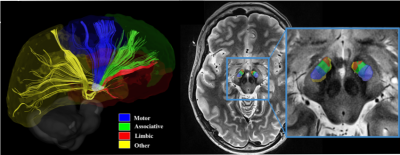
Deep brain stimulation (DBS) in the subthalamic nucleus (STN) is an effective therapy for the motor symptoms associated with Parkinson’s disease (PD). While some have argued that equivalent motor outcomes can be achieved with a DBS lead placed within a 6mm diameter cylinder of an atlas-estimated “middle” of the STN1, others have argued that the lead should be placed within the sensorimotor territory of the STN2,3. These discrepancies are likely due to the fact that the vast majority of studies rely heavily on the use of anatomical templates which do not take into account inter-patient variability. In fact, a recent study taking advantage of the increased signal-to-noise ratio and resolution from 7T MRI found that the size, shape and orientation of the STNs differed across patients4. Further, Plantinga et al.5 used tractography-based parcellation of 7T using patient-specific data and found a reproducible organizational pattern of the functional territories of the STN. To date, however, clinical validation of such image-based parcellations remained specific to the motor territory and were never tested or confirmed for regions outside of it, namely, the associative and limbic regions of the STN.Here, we report of a unique circumstance where a DBS patient following an electrode implantation developed severe depressive side effects and mild motor benefit. Electrode revision that repositioned the electrode posteriorly alleviated the mood side effects while greatly improving motor signs. These clinical findings are explained by using a patient-specific, 7T tractography-based, parcellation of the STN.
Methods
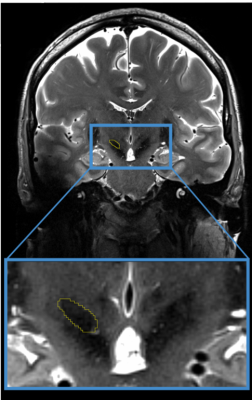
Prior to undergoing her initial awake bilateral STN DBS surgeries with micro-electrode recording, a 52-year-old right-handed woman with a 14-year history of PD was scanned at 7T MRI. Scanning protocol included a 0.4x0.4x1mm T2, a 0.4x0.4x0.8mm SWI, 0.6mm isotropic T1 and 1.5mm isotropic DWI (54directions, b=1500, AP and PA acquisition)4,5. The patient’s STN, along with other structures, was manually segmented using the contrast from the T2 and the SWI (Figure 1). Cortical masks representing motor, limbic, associative and “other” regions were non-linearly brought to native T1 space and further tailored to the patient’s anatomy by only keeping voxels which intersected with the patient’s grey matter mask (dilated once). After distortion, motion and eddy correction, tractography-based parcellation of the patient's STN was performed. Then, post-operative computed tomography data (resolution=0.3x0.3x0.6mm) was used to determine the final location of the electrode with respect to the patient’s own anatomy.Results
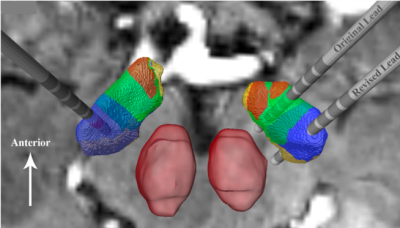
Following the initial surgery, DBS programming revealed that the stimulation with the left electrode resulted in good motor symptom suppression on the right body while stimulation with the right electrode induced depressive side effects and only mild improvement in the left body (31% improvement). Parcellation of the STN followed the same reproducible pattern observed in Plantinga et al.5 whereby the motor territory was located in the posterior part of the STN followed anteriorly by the associative and the limbic regions (Figure 2). Upon reviewing the imaging-based reconstruction of the electrode location with respect to the parcellation, it was observed that the right electrode was located in the anterior portion of the STN, where the associative and limbic sections of the structure reside (Figure 3). The right electrode was subsequently revised and the lead was placed more posteriorly in the STN, which correspond to the motor territory based on the parcellation analysis (Figure 3). Following the revision surgery, upon programming her DBS system, the patient did not report any acute mood side-effect and the left body motor improvements increased to 92%. Figure 3 confirms that the revised lead was correctly placed in the motor territory without infringing on the limbic region and showed good correspondence between the clinical observations and the imaging-based methods.Discussion and Conclusion
Using 7T MRI, we provide a first-in-kind within-patient validation that patient-specific segmentation and parcellation has the capability to accurately depict the location of different anatomical subregions of the STN, allowing a correlation of stimulation clinical outcomes for select regions of the STN. We show that when our imaging-based reconstruction indicates that the DBS lead is implanted in limbic and associative areas, stimulation induces mood side effects, while when the reconstruction indicates the lead is placed within the sensorimotor territory, greater motor improvement occurs without the associated mood side effects. The significant improvement in motor benefit seen after repositioning the lead in the sensorimotor territory is in agreement with previous studies that have suggested optimal motor benefit with stimulation of the dorsolateral motor STN3. This result, however, is in stark contrast to other reports who, using template anatomy, have argued that similar outcomes can be produced with stimulation anywhere within the STN1,6, or that the greatest motor improvements are seen in the more anterior electrode locations, closer to or in the associative territory7. This discrepancy may reflect the fact that these studies lack the visualization tools that would allow for accurate determination of postoperative lead location with respect to individualized patient STN anatomy. Using 7T MRI, which was recently FDA-approved for clinical use, we present a tool that may be able to answer previously unresolved questions in the field of DBS, and by its very nature will bring us one step closer to personalized DBS medicine.Acknowledgements
This work was supported in part by R01-NS081118, R01-NS113746, P50-NS098573, P30-NS076408 and P41-EB027061 and the University of Minnesota Udall center P50NS098573.References
1. McClelland, S., 3rd, et al., Subthalamic stimulation for Parkinson disease: determination of electrode location necessary for clinical efficacy. Neurosurg Focus, 2005. 19(5): p. E12.
2. Herzog, J., et al., Most effective stimulation site in subthalamic deep brain stimulation for Parkinson's disease. Mov Disord, 2004. 19(9): p. 1050-4.
3. Wodarg, F., et al., Stimulation site within the MRI-defined STN predicts postoperative motor outcome. Mov Disord, 2012. 27(7): p. 874-9.
4. Duchin, Y., et al., Patient-specific anatomical model for deep brain stimulation based on 7 Tesla MRI. PLoS One, 2018. 13(8): p. e0201469.
5. Plantinga, B.R., et al., Individualized parcellation of the subthalamic nucleus in patients with Parkinson's disease with 7T MRI. Neuroimage, 2018. 168: p. 403-411.
6. Kasasbeh, A., et al., Lack of differential motor outcome with subthalamic nucleus region stimulation in Parkinson's disease. J Clin Neurosci, 2013. 20(11): p. 1520-6.
7. Welter, M.L., et al., Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology, 2014. 82(15): p. 1352-61.
Figures