2001
Hippocampus subfield segmentation reliability is improved by non-linear realignments between scan-repetitions at 7T and 3T.1The University of Queensland, Brisbane, Australia, 2QIMR Berghofer Medical Research Intstitute, Brisbane, Australia, 3Queensland University of Technology, Brisbane, Australia, 4CSIRO Health and Biosecurity, Brisbane, Australia, 5CSIRO Data61, Sydney, Australia
Synopsis
MR image quality is affected greatly by participant movement. Anatomical image quality can be improved by acquiring consecutive anatomical scans and combining them to boost SNR and sharpness. We aimed to find the optimal combination method of three repetitions of high-resolution turbo spin-echo (TSE) scans, commonly used for hippocampus subfield segmentation. We used non-linear realignment between the TSE repetitions in a range of participants at 3T and 7T and found that image segmentation reliability and sharpness were higher for the non-linear realignment technique, as compared to linearly realigned, and averaged methods. Hippocampus image segmentation greatly benefits from this technique.
Introduction
Increasing spatial resolution for MRI improves segmentation of various tissue types or anatomical structures1. Large movement artefacts increase partial volume effects and decrease interpretability of anatomical scans for clinicians and automatic/manual segmentation protocols. Therefore, there is a need to acquire multiple repetitions of a single sequence to improve signal-to-noise ratio (SNR), which leads to longer scan times. This includes dedicated Turbo-Spin Echo (TSE) scans for hippocampus segmentation. With longer acquisition times comes a higher propensity for participant movement, which can deteriorate image quality leading to increased costs and unusable data, and patients with neurodegenerative disorders, elderly, young, and highly anxious participants are particularly likely to move in the scanner.3Image segmentation is especially affected by MRI movement artefacts. Therefore, in order to ameliorate movement artefacts, improve image segmentation reliability, and increase sharpness and SNR, we implemented a non-linear realignment technique to improve hippocampus subfield segmentation at 7T and 3T in healthy controls, Motor Neuron Disease (MND) patients, and adolescent participants. Inherently, segmentation algorithms rely on high sharpness and SNR for registration. Therefore, we chose to assess segmentation reliability based on registration success and sharpness.
Methods
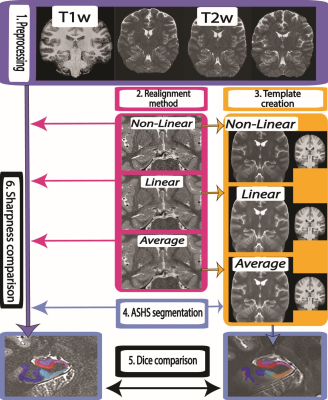
In order to test the robustness of the technique on a range of participants, we acquired a 2D TSE sequence thrice per participant at both 7T (0.4x0.4x0.8mm3) and 3T (0.5 x 0.5 x 1.0mm3) acquired orthogonal to the hippocampus proper in four samples of participants: at 7T, 11 patients diagnosed with MND (Age, M=59.36, SD=7.65), 11 age-matched control participants (HCs, M=60.23, SD=7.65), and 29 young healthy participants (YHPs, M=26.31, SD=0.66) and at 3T, 24 adolescent participants (details) An anatomical whole-brain T1w MP2RAGE4,5 (.9mm iso) was also acquired (7T: TR/TE/TIs=4300ms/2.5ms/840,2370ms; 3T (TR / TE / TIs = 4000ms / 2.9ms / 700ms / 2220 ms).Pre-processing of images included isotropic resampling to 0.3mm3 (0.5mm for 3T), bias field correction and intensity normalisation6. To examine registration precision and segmentation reliability, we compared three separate methods (1) Non-Linear, (2) Linear, and (3) Average. The Non-Linear method involved creating a minimum deformation average template using ANTs SyN registration7,8 (Fig 1.) The three repetitions were iteratively deformed to a synthetic template as to not bias any repetition in the realignment process. For the Linear method, we first used FSL9 to concatenate images in time, then estimating the registrations between the repetitions with MCFLIRT10, then averaging the 4-dimensional images. The Average method involved taking the scanner-outputted arithmetic mean.
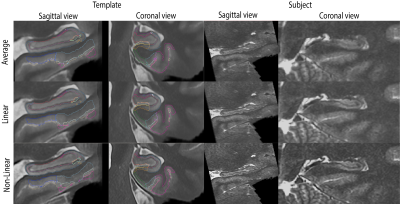
Intuitively, image registration might benefit from by images with higher sharpness, better SNR, and more refined details and therefore, registration consistency would be increased between participants if the realignment method is more reliable. To assess the three methods, we co-registered each participant from each group to group-specific templates using ANTs, for each method (Non-Linear, Linear, Average, see Figure 1). This yielded 12 templates (e.g., MND-Linear, Healthy controls-Non-Linear, adolescents-Average etc.). Then, each of these templates were labelled using Automatic Segmentation of Hippocampus Subfields (ASHS)3. Participants’ scans were also labelled using ASHS in a common space (Figure 2). The resulting templates and individual scans were compared using Dice overlaps11 between the group template and the individual for each method (Non-Linear, Linear, Average). In this way, we could determine the resulting segmentation reliability of each method. The sharpness of each image was also compared for each of the three methods by calculating the median of the derivative of a Gaussian applied to the TSE at 1mm FWHM as described in Fonov & Collins (2018)12.
Results
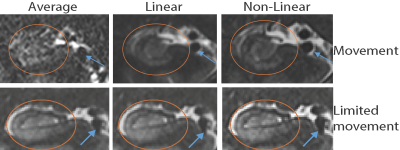
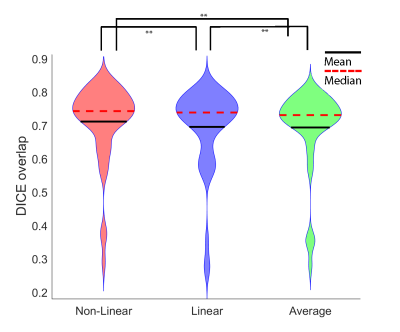
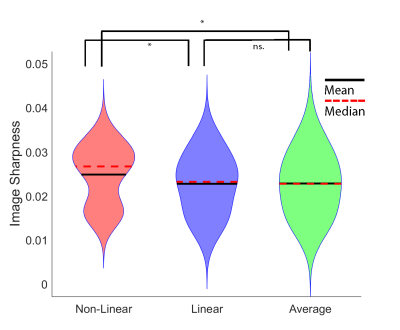
Fig. 3 shows the results of each realignment method on two subjects, one with excessive movement (as rated by three raters) and one with limited movement. When collapsing across all participant groups, Dice overlaps for the Non-Linear method were significantly higher than for Linear and Average Fig 3; N=51; ** = ps < 0.001, Wilcoxon rank sum tests).When comparing sharpness between the three methods (Figure 5), we found differences between all three groups (p < 0.001), with Non-Linear showing higher sharpness than Linear and Average (** = ps < 0.05). No difference was found between Linear and Average. (p = .982, Wilcoxon rank sum test). 3T results found the same pattern of results for sharpness.
Discussion and conclusion
We examined the effect of realignment strategies for multiple repetitions of high-resolution images of the hippocampus at both 3T and 7T and found a Non-Linear realignment strategy produces more reliable and sharper segmentations than a Linear or Average method. Non-Linear realignment therefore improves the robustness of brain image segmentation and mitigates the effects of motion artefacts in high-resolution anatomical MRI.Hippocampus subfield segmentation can benefit from non-linear registrations when participant motion between scans is an issue. It is proposed that due to the high computational cost, non-linear realignment be used judiciously in participants with high inter-scan motion, as determined by metrics such as sharpness or qualitative assessment.
Acknowledgements
The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Centre for Advanced Imaging, The University of Queensland. MB acknowledges funding from Australian Research Council Future Fellowship grant FT140100865. This research was undertaken with the assistance of resources and services from the Queensland Cyber Infrastructure Foundation (QCIF). The authors gratefully acknowledge Aiman Al Najjer and Saskia Bollmann for acquiring data. The data acquisition was partly funded by a study from the Cooperative Research Centre for Mental Health. Data for the 24 adolescent participants was provided by the Queensland Adolescent Twin Brain project, funded by the NHMRC (APP1078756).References
1. Thomas, B. P. et al. High-resolution 7T MRI of the human hippocampus in vivo. J. Magn. Reson. Imaging JMRI 28, 1266–1272 (2008).
2. Kochunov, P. et al. Retrospective motion correction protocol for high-resolution anatomical MRI. Hum. Brain Mapp. 27, 957–962 (2006).
3. Yushkevich, P. A. et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp. 36, 258–287 (2015).
4. Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 49, 1271–1281 (2010).
5. O’Brien, K. R. et al. Robust T1-Weighted Structural Brain Imaging and Morphometry at 7T Using MP2RAGE. PLOS ONE 9, e99676 (2014).
6. Tustison, N. J. et al. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
7. Avants, B. B. et al. The Optimal Template Effect in Hippocampus Studies of Diseased Populations. NeuroImage 49, 2457 (2010).
8. Avants, B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric Diffeomorphic Image Registration with Cross-Correlation: Evaluating Automated Labeling of Elderly and Neurodegenerative Brain. Med. Image Anal. 12, 26 41 (2008).
9. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. NeuroImage 62, 782–790 (2012).
10. Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 17, 825–841 (2002).
11. Dice, L. R. Measures of the Amount of Ecologic Association Between Species. Ecology 26, 297–302 (1945).
12. Fonov, V. & Collins, D. L. Comparison of different methods for average anatomical templates creation: do we really gain anything from a diffeomorphic framework? (2018) doi:10.1101/277087.
Figures