1999
Anatomical NeuroImaging with Single and Parallel Transmission at Ultra-high Field: A Comparison of Image Quality and User Experience1University of Southern California, Los Angeles, CA, United States
Synopsis
B1+ field inhomogeneity is an ongoing issue that plagues whole-brain anatomical imaging at ultra-high field, hindering its translation to the clinic. This work evaluates and compares the latest commercially available options for RF shimming using NOVA 1Tx/32Rx and parallel 8Tx/32Rx coil systems. Various configurations including the use of dielectric pads for 1Tx, or volume-selective and patient-specific RF shimming with pTx, were systematically compared with the purpose of evaluating benefits for clinically relevant anatomical imaging. Experimental results showed a consistent significant drop in the B1+ field map in the temporal lobe, which was ameliorated using pTx.
Purpose
Ultra-high field (UHF) MR imaging, where the main magnetic field (B0) strength is greater than or equal to 7T, has been shown to provide benefits such as higher spatial resolution, improvements in signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR), and improved temporal resolution for dynamic processes. However, whole-brain neuroimaging at 7T also presents significant challenges with B1+ inhomogeneity that may hinder clinical adoption of UHF imaging due to insufficient diagnostic image quality. The introduction of parallel transmission (pTx) was previously proposed to alleviate RF inhomogeneities1, 2. In this work, five commercially available solutions provided by Siemens and NOVA Medical systems are systematically compared for benefits in anatomical imaging including: (1) combined mode single transmission 1Tx+32Rx NOVA coil system [Nova Medical, Inc., Wilmington, MA] with and (2) without the use of dielectric pads3, and pTx mode 8Tx+32Rx NOVA coil system (Nova Medical, Inc.) operating in (3) quadrature (circular polarization-like/TrueForm), (4) patient-specific, and (5) volume-selective RF shimming configurations, where the selected volume is focused on the commonly problematic temporal lobe region.Methods
All experimental data were acquired using a 7T Terra whole-body MR system equipped with 8-channel pTx array capability [Siemens Healthcare, Erlangen, Germany]. Five healthy volunteers (1 female, 27±2.3 years) were either fitted with 3D-printed helmets or securely padded into the head coil for motion prevention (Caseforge, Berkeley, CA) and scanned using both pTx and 1Tx combined modes in a single 2-hour session. Switching between the FDA-approved 1Tx combined mode and research-only pTx mode typically required an average time of about 10 minutes. First-level controlled SAR mode was employed for 3D structural imaging sequences to ensure FDA guidelines (3.2W/kg on head) were met. RF field mapping was performed using a Turbo Flash sequence with the following parameters: FOV=256x256mm, TR/TE=4000/1.73ms, FA=5°, BW=440Hz/Px, resolution=4.0mm3. Sagittal T1-weighted MPRAGE images were acquired with the following parameters: resolution=0.7mm3 isotropic, GRAPPA=2, TR/TE/TI=2200/2.95/1050ms, scan time ~6.5min. Sagittal T2-weighted SPACE images were acquired with the following parameters: resolution= 0.6mm3 isotropic, GRAPPA=3, TR/TE=2140/221ms, scan time ~11min. All configurations were matched to the highest SAR-limit allowed input voltage and had the same sequence parameters. Current commercially available coil array configurations were evaluated based on B1+ homogeneity, signal intensity and coverage, repeatability across different subjects, as well as ease of operation for clinical applicability and performance in future neurology patient studies focusing on diagnostic image quality (SNR, CNR).Results and Discussion
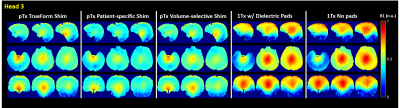
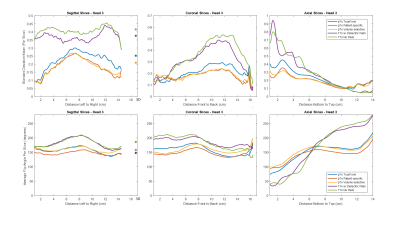
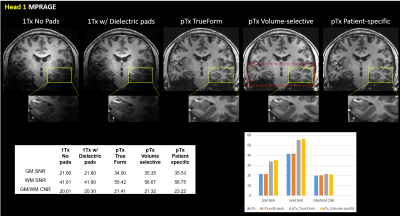
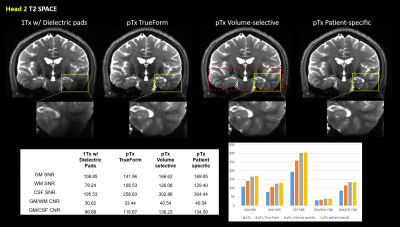
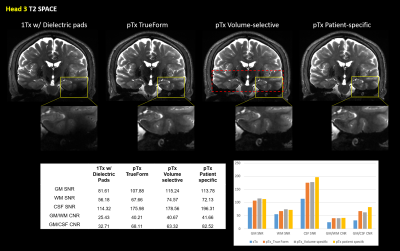
Figure 1 shows qualitative comparisons of measured in vivo B1+ field distributions for three views between the pTx system using TrueForm, Patient-specific, and medial-temporal lobe focused Volume-selective (dashed red box) B1 shim modes, as well as 1Tx combined mode with and without the use of dielectric pads. A significant drop of the B1+ field intensity was observed in the temporal lobe and cerebellum for the combined mode 1Tx NOVA coil system. While the addition of dielectric pads slightly improves B1+ field inhomogeneity, the temporal lobes still suffer from signal dropout. In contrast, this medial temporal region exhibits improved homogeneity in all configurations with the pTx NOVA coil system, an observation which was expected due to the expanded coil coverage. Figure 2 shows a quantitative experimental analysis for Head 3. The mean coefficient of variation (CV=std/mean) of measured B1+ field was 0.21 when using either patient-specific or volume-selective B1 shim mode with the pTx coil system, indicating improved RF homogeneity when compared to the other configurations (0.25 for pTx TrueForm, 0.38 for 1Tx with dielectric pads, and 0.42 for 1Tx without pads). When using the same input voltage, the mean B1+ intensity over the whole head decreases by ~20% for all subjects with patient-specific RF-shimming, compared to the clinically used 1Tx with dielectric pads (Figure 2).Concerning application of in vivo anatomical imaging, the pTx coil system demonstrates significant benefits for clinical use. Figure 3 displays the T1-weighted MPRAGE images of Head 1 for both 1Tx and pTx coil system configurations. Patient-specific pTx B1 shimming enabled higher SNR and improved CNR between gray (GM) and white matter (WM) in the medial-temporal region. Figures 4 and 5 display the T2-weighted SPACE images for Heads 2 and 3, respectively. As shown in the magnified insets focusing on the hippocampus and parahippocampal gyrus/temporal lobe in Figure 4, the signal dropout due to B1 field inhomogeneity is alleviated in pTx mode, with especially improved qualitative delineation of hippocampal structures when utilizing patient-specific or volume-selective shimming configurations. Furthermore, in one subject, pTx shimming enabled the detection of extracerebral structures like the cochlea (Figure 5). Interestingly, T2-weighted SPACE images exhibited unexpected contrast near the brainstem due to an accentuated CSF pulsation artifact in the basal cisterns. This artifact was mitigated with the application of flow compensation in addition to pTx RF shimming, revealing fine structures such as the abducens nerve with the restored high contrast between tissue and cerebrospinal fluid (CSF).
Conclusion
The comparison of commercially available coil configurations showed that the patient-specific shimming, achieved through parallel transmission, can substantially improve RF homogeneity in anatomical neuroimaging. While further developments are needed to limit SAR deposition for 3D T1 or T2 imaging, using pTx can address homogeneity issues and directly benefit diagnostic imaging of important structures related to dementia and other neurodegenerative diseases.Acknowledgements
This work was supported by National Institutes of Health grants UH2-NS100614, S10-OD025312, K25-AG056594 and P41-EB015922. This work was also supported by American Heart Association grant 16SDG29630013.References
1. Ibrahim TS, Lee R, Baertlein BA, Kangarlu A, Robitaille P-ML. Application of finite difference time domain method for the design of birdcage rf head coils using multi-port excitations. Magnetic Resonance Imaging. 2000;18:733-742
2. Hoult DI. Sensitivity and power deposition in a high-field imaging experiment. Journal of Magnetic Resonance Imaging. 2000;12:46-67
3. Yang QX, Mao W, Wang J, Smith MB, Lei H, Zhang X, et al. Manipulation of image intensity distribution at 7.0 t: Passive rf shimming and focusing with dielectric materials. Journal of Magnetic Resonance Imaging. 2006;24:197-202
Figures