1998
Single and repeated ketamine treatment induces perfusion changes in sensory and limbic networks in major depressive disorder1UCLA, Los Angeles, CA, United States, 2University of Southern California, Los Angeles, CA, United States
Synopsis
Ketamine infusion therapy is now well-replicated to produce fast-acting antidepressant effects in patients with major depressive disorder (MDD). However, brain systems-level hemodynamic changes in response to single and repeated ketamine treatment remain uncharacterized. Using advanced MB pCASL MRI we examined CBF changes occurring with single and repeated ketamine treatment. Initial changes in blood flow were observed in the posterior cingulate and precuneus and primary and higher order visual areas. However, repeated exposure to ketamine engaged deeper limbic structures and the insula. Findings demonstrate that ketamine treatment perturbs distinct functional networks including sensory and limbic regions
INTRODUCTION
Neuroimaging research continues to provide important insights into the pathophysiology of major depressive disorder (MDD), and the physiological basis of antidepressant response1. However, response to standard antidepressants is modest and protracted, and one-third of patients defined as having treatment resistant depression (TRD) are expected to fail ≥ 2 treatment trials2.Knowledge of rapid response mechanisms is thus pivotal for advancing more effective interventions. Ketamine, an antagonist of N-methyl-D-aspartate (NMDA) receptors, has long been used as an anesthetic. At subanesthetic doses, ketamine is now also well-replicated to produce acute and robust antidepressant effects3. Still, the downstream antidepressant effects of ketamine on functional neurocircuitry remain uncertain. Arterial spin labelling (ASL) perfusion MRI provides a quantitative measure of regional cerebral blood flow (rCBF) and is shown as sensitive for detecting effects of clinically effective doses of marketed drugs. ASL MRI can thus offer novel understanding of the brain systems-level antidepressant effects of ketamine. Leveraging advanced multiband (MB) pseudo-continuous ASL (pCASL) imaging4, in the current investigation we compared global and rCBF measured at rest in TRD patients at baseline and 24 hours after receiving single, and four serial infusions of subanesthetic ketamine. We hypothesized that ketamine infusion would associate with increased rCBF in sensory and association cortices, and decreased rCBF in subcortical limbic regions5.METHODS
Participants included 18 healthy controls (HC) and 22 DSM–5 defined individuals with MDD, who met criteria for TRD (i.e., failed ≥ 2 adequate antidepressant trials and had been continuously depressed for ≥ 6 months, all 20 – 64 years of age). All TRD subjects received a series of four ketamine treatments and were followed prospectively during treatment. Imaging and clinical assessments occurred at three timepoints: 1) initial baseline (TP1) occurring within one week of the first treatment; 2) 24 hours after the first ketamine infusion (TP2) and; 24 to 72 hours after the last ketamine infusion (TP3). All subjects provided written informed consent following procedures approved by the University of California, Los Angeles (UCLA) Institutional Review Board (IRB). Imaging data was acquired on a Siemens 3T Prisma MRI system at UCLA’s Brain Mapping with a 32-channel head coil. Acquisition sequences were identical to those used by the Human Connectome Project (HCP) Lifespan studies for Aging and Development (https://www.humanconnectome.org). Structural scans consisted of a T1-weighed (T1w) multi-echo MPRAGE (voxel size (VS)=0.8mm isotropic; repetition time (TR)=2500ms; echo time (TE)=1.81:1.79:7.18ms; inversion time (TI)=1000ms; flip angle (34)=8.0o; acquisition time (TA)=8:22min) and a T2-weighted (T2w) acquisition (VS=0.8mm isotropic; TR=3200ms; TE=564ms; TA=6:35min), both with real-time motion correction6. MB-EPI pCASL data (MB acceleration factor = 6, 60 slices with an isotropic resolution of 2.5 mm, TR/TE= 3.58/19 ms, labelling duration = 1500 ms, 5min 29 sec duration) as described in Harms et al7 was acquired to quantify CBF changes. For pCASL calibration, two additional M0 scans were collected using a proton density scan with the same readout as MB-EPI pCASL acquisition. All image processing and analysis was carried out using the FMRIB software library (FSL 6.0.1)8 and the Connectome Workbench tool (https://www.humanconnectome.org/software/connectome-workbench, version 1.3.2). FSL’s topup tool corrected for MB-EPI pCASL distortions using the spin-echo images. After distortion correction, the Oxford_asl tool9 computed calibrated CBF (ml/100g/min) maps. Regional effects of ketamine were confirmed with permutation testing (n=5000) using paired t-tests of calibrated CBF maps for time points examined pairwise (TP1 and TP2, TP2 and TP3, TP1 and TP3). Statistical thresholds were set at threshold-free cluster estimates (TFCE) p<0.05.RESULTS
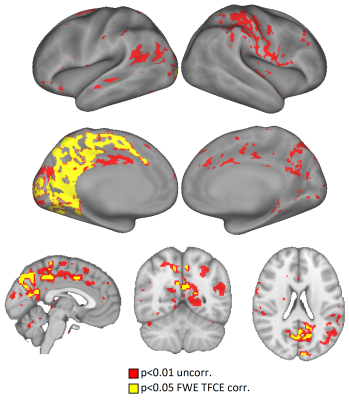
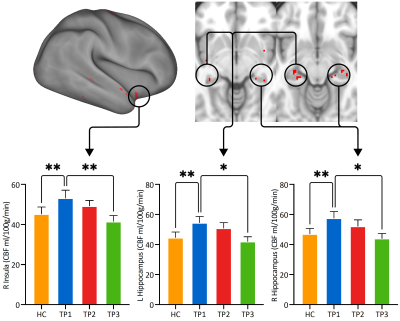
Age and sex did not significantly differ between HC and MDD. The paired t-test comparing baseline (TP1) and first ketamine infusion (TP2) revealed post-treatment increases in rCBF in parallel analysis of both CIFTI (optimized for the cortex) and volume (optimized for subcortical regions) data (Figure 1). Amongst widespread mean increases in CBF (shown in red at p<0.01 uncorrected), significant increases (shown in yellow at p<0.05 FWE (family wise error corrected) were observed in the mid and posterior cingulate and proximal association areas encompassing the paracentral lobule, cuneus, precuneus, and other higher-order visual association regions including the fusiform. A significant decrease in rCBF was observed for the paired t-test comparing TP1 and TP3 in the bilateral hippocampus and right insula (Figure 2, p<0.01, uncorrected), although clusters did not survive TFCE FWE thresholdsDISCUSSION
Our results show that changes in rCBF are present 24 hours after treatment demonstrating neuroplasticity occurs beyond the immediate effects of ketamine on glutamate signaling. Our findings also support that repeated low-dose ketamine therapy leads to neurofunctional plasticity in a temporally specific regional pattern. Specifically, results show neuroplasticity in primary and higher-order visual areas, as well as the posterior cingulate and precuneus following initial ketamine treatment. The changes in perfusion observed in the bilateral hippocampus and right insular following serial treatment suggest that repeated exposure to ketamine may be optimal for perturbing subcortical circuits. Regional changes in CBF with treatment occurred the direction of control subjects.CONCLUSION
Findings demonstrate that neurophysiological changes occurring with single and repeated ketamine treatment follow both a regional and temporal pattern including sensory and limbic regions.Acknowledgements
This work was supported by the National Institute of Mental Health of the National Institutes of Health (Grant Nos. MH110008 [to KLN and RE], MH102743 [to KLN], and the Muriel Harris Chair in Geriatric Psychiatry (to RE)). This research was additionally supported by the UCLA Depression Grand Challenge, support for which is provided by the UCLA Office of the Chancellor and philanthropy.References
1. Wei, W., et al., Trajectories in Cerebral Blood Flow Following Antidepressant Treatment in Late-Life Depression: Support for the Vascular Depression Hypothesis. J Clin Psychiatry, 2018. 79(6).
2. Nemeroff, C.B., Prevalence and management of treatment-resistant depression. J Clin Psychiatry, 2007. 68 Suppl 8:17-25.
3. Zarate, C.A., Jr., et al., A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry, 2013. 74(4):257-64.
4. Li, X., et al., Theoretical and experimental evaluation of multi-band EPI for high-resolution whole brain pCASL Imaging. Neuroimage, 2015. 106:170-81.
5. Carlson, P.J., et al., Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry, 2013. 73(12):1213-21.
6. Tisdall, M.D., et al., Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med, 2012. 68(2):389-99.
7. Harms, M.P., et al., Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage, 2018. 183:972-984.
8. Smith, S.M., et al., Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 2004. 23 Suppl 1:S208-19.
9. Groves, A.R., M.A. Chappell, and M.W. Woolrich, Combined spatial and non-spatial prior for inference on MRI time-series. Neuroimage, 2009. 45(3):795-809.
Figures