1997
Ketamine modulation of Cerebro-Cerebellar Circuitry During Response Inhibition in Major Depression1Neurology, Ahamason-Lovelace Brain Mapping Center, UCLA, Los Angeles, CA, United States, 2Radiology, Center for Translational Imaging, Northwestern University, Chicago, IL, United States, 3Psychiatry and Biobehavioral Sciences, UCLA, Los Angeles, CA, United States
Synopsis
Treatments for major depressive disorder (MDD) target communication between large-scale cortical networks and the cerebellum to improve cognitive control [2]. Here, we examined how repeated-ketamine perturbs cerebro-cerebellar circuitry during response-inhibition using a NoGo/Go task (43 MDD-patients receiving ketamine, 31 controls). We implemented a psychophysiological-interaction analysis to investigate ketamine-related changes in connectivity between cerebellum-regions and cortical-networks and respective nodes. Results showed significant decreases in connectivity between a cerebellum-dorsal-attention region and the default-network. A time-by-response interaction for the somatomotor-network was observed in treatment-responders only. Results support ketamine modulates cerebello-cerebro circuitry, which may be of impact for identifying new biomarkers of MDD response.
Introduction
Patients with major depressive disorder (MDD) have impaired cognitive control and mood regulation, which have been attributed to abnormal communication between large-scale functional brain-networks [1]. Based on evidence of cortico-cerebellar and cerebello-thalamo-cortico loops, the cerebellum is now understood to play a critical role in the communication between major large-scale cortical networks [2]. The cerebellum’s contribution to cognitive control is supported by observations of poor performance during response-inhibition tasks in subjects with cerebellar impairments, and is found to influence post-error processing through communication with the prefrontal cortex and anterior cingulate via the thalamus [3,4]. Ketamine therapy is shown to induce rapidly-acting antidepressant effects in patients with MDD [5]. Despite initial evidence suggesting ketamine alters resting-state functional-connectivity in large-scale networks [6,7], no study to date has investigated how ketamine perturbs cerebello-cerebro circuitry during cognitive control in MDD. Thus, using a response-inhibition Go/NoGo activation-task, we performed psychophysiological-interactions (PPI) analysis to examine correlations in brain activity between three functionally distinct cerebellar areas and five major functional networks to compare changes in cerebello-cerebro circuitry before and after patients received serial-ketamine treatment, and differences with controls.Methods
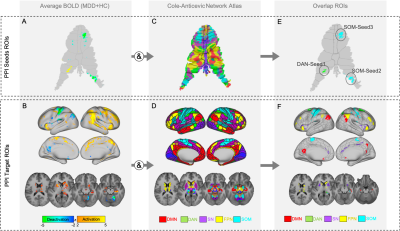
MDD patients (n=43, mean age=39.19, 42.3% female) received four 0.5 mg/kg infusions of ketamine administered 2-3 times weekly. MRI and clinical data were collected before (T1), and 24 hours after the first (T2) and fourth (last) ketamine infusions (post-treatment: T3). Healthy controls (HC) (n=31, mean age=35.78, 61.3% female) completed one MRI session (T1). A T1w structural (TE=4.6ms, TR=9.9ms, voxel size=0.8x0.8x0.8mm3, FA=2o) and task-functional MRI scan (TE=37ms, TR=800ms, voxel size=2x2x2mm3, FA=52o, MB accl. factor=8, acquisition time=6min) using a response inhibition Go/NoGo paradigm [8] were acquired from all subjects at each time point. Functional images were preprocessed using the HCP minimal preprocessing pipeline [9], followed by FIX denoising [10], and analysis was performed in CIFTI space [9]. Cerebellum surfaces were mapped to the Colin-cerebellum atlas using Workbench1.3.2 [11]. A general-linear-model (GLM) was fitted to each fMRI session, and PALM [12] estimated average BOLD activity for the NoGo-Go contrast across all participants at baseline. Using a network atlas [13] we targeted five networks implicated in MDD: default mode (DMN), frontoparietal (FPN), dorsal-attention (DAN), salience (SN) and somatomotor (SOM) networks. The regions that overlapped between the NoGo-Go functional-contrast map and the five networks, were used to define seeds- and target-PPI regions. The regions that overlapped in the cerebellum were defined as PPI-seeds; the regions that overlapped in the cerebrum were defined as PPI-target ROIs (Figure1). Extracted seeds included: cerebellum-lobuleVIIb, associated with the DAN (DAN-seed1); and two cerebellum-regions associated with the SOM (SOM-seed2:lobuleVIIIa and SOM-seed3:lobuleV). For post-hoc analyses, 4-5 key network hubs for each network were selected to additionally investigate within network connectivity. To investigate how each cerebellar-seed modulates each of the five cerebral large-scale networks and their respective nodes, GLM-PPI models were fitted to each subject and session and average PPI beta scores were extracted from the target-ROIs. This analysis led to one average PPI-score for each network, and each respective node per subject and timepoint. Higher-order analysis were conducted in SPSS [14] using general linear mixed models (GLMMs) to examine main effects of time (T1, T2 and T3), ketamine response (responders and non-responders using a >50% change in mood scores over treatment to define response), and time-by-response interactions for the five networks and their respective nodes. A Bonferroni corrected alpha of p<0.017 (0.05/3 PPI seeds) was used as the statistical threshold. For significant target-ROIs, post-hoc analysis compared HC with MDD at baseline.Results
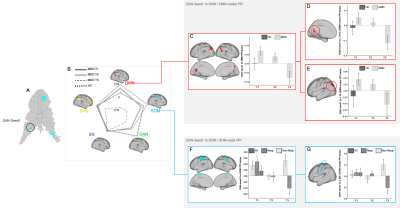
The GLMMs revealed a significant effect of time for PPI-connectivity between DAN-seed1 and the DMN (F(2)=4.72,p=0.01) (connectivity decreased), and a time-by-response interaction between DAN-seed1 and the SOM (F(2)=4.44,p=0.013) (connectivity decreased at T3 for responders only). Post-hoc analysis of PPI-connectivity between the DAN-seed1 to particular DMN nodes revealed an effect of time for the left posterior cingulate (F(2)=5.70,p=0.005), and left inferior parietal cortex, (F(2)=3.10,p=0.05). Post-hoc analysis of PPI-connectivity between DAN-seed1 and SOM nodes showed a time-by-response interaction for the left motor cortex (MC) (F(2)=5.62,p=0.005). There were no additional significant PPI effects for the remaining seeds and networks (Figure2).Discussion
Results showed a reduction in PPI-connectivity between cerebellar attention-related functional areas (DAN-seed1) and the DMN, after ketamine infusion. These findings are in accordance with prior observations suggesting that MDD is characterized by aberrant communication between the DMN (which relates to internally-directed attention) and task-positive networks such as the DAN [9], and support the cerebellum modulates cortical activity during response-inhibition processes. In particular, closed loops between the parietal-lobe and cerebellum-lobuleVII are known to be involved in visuomotor control as well as in higher-order functional processing related to goal directed-attention [8]. Increased coupling between the cerebellum and MC has been previously associated with compensatory pathways related with reduced coupling between the motor-cerebellum and the dorsolateral-prefrontal cortex [15], which may explain the reduction in connectivity between DAN-seed1-cerebellum with the MC for ketamine responders only.Conclusion
Using novel computational-connectomics methods, this study demonstrates that ketamine affects distinct cortico-cerebellar circuitry during response inhibition and that changes differ in treatment-responders. Findings add new knowledge regarding the systems-level effects of ketamine and may help identify new biomarkers of response to advance more effective personalized treatments for MDD.Acknowledgements
This work was supported by the National Institute of Mental Health of the National Institutes of Health (Grant Nos. MH110008 [to KLN and RE], MH102743 [to KLN], and the Muriel Harris Chair in Geriatric Psychiatry (to RE)). This research was additionally supported by the UCLA Depression Grand Challenge, support for which is provided by the UCLA Office of the Chancellor and philanthropy.References
[1] Menon, Menon. Large-scale brain networks and psychopathology: a unifying triple network model. TICS, 15(10): 483-506, 2011. [2] Buckner, R. L., et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology.; 106: 2322–2345, 2011. [3] Ramnani, Narender. Frontal Lobe and Posterior Parietal Contributions to the Cortico-cerebellar System. The Cerebellum, 11(2): 366–383, 2011. [4] Miquel, M., et al. A Working Hypothesis for the Role of the Cerebellum in Impulsivity and Compulsivity. Frontiers Behav. Neurosci.; 13, 2019. [5] Duman R. S, et al. Synaptic plasticity and depression: New insights from stress and rapid acting antidepressants. Nat Med.; 22(3): 238-249, 2016. [6] Evans, J. W., et al. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biological Psychiatry; 84(8):582-590, 2018. [7] Anticevic, Alan, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. PNAS, 109(41): 16720-16725, 2012. [8] Bookheimer, Susan Y. et al., The Lifespan Human Connectome Project in Aging: An overview. Neuroimage, 185: 335–348, 2019. [9] Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage.; 80:105–124, 2013. [10] G. Salimi-Khorshidi, et al. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. NeuroImage, 90:449-68, 2014. [11] Connectome Workbench 1.3.2: https://www.humanconnectome.org/software/connectome-workbench [12] Winkler AM, et al. Permutation inference for the general linear model. NeuroImage; 92:381-397, 2014. [13] Ji, Jie Lisa, et al., Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage, 185: 35-57, 2019. [14] IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [15] Alalade, Emmanuel, et al., Altered Cerebellar-Cerebral Functional Connectivity in Geriatric Depression. Plos one, 6(5), 2011.Figures