1994
Smaller Hippocampal Subfields Associate with Poorer Logical Memory Performance in a Large Sample of MDD Patients1Radiology, Huaxi MR Research Center (HMRRC), Sichuan University, Chengdu, China
Synopsis
Smaller hippocampus is a consistent finding in studies of major depressive disorder (MDD), and subfield-level anatomic analyses could provide new insights to the role of hippocampal deficit in MDD. In the current study, we recruited a relatively large sample of MDD patients and demonstrated that (1) “core structures” including CA and dentate gyrus are more anatomically involved in MDD than other structures in hippocampus; (2) hippocampal tail might be important in MDD, but the relationship between hippocampal tail and MDD could be complex; (3) smaller hippocampus could contribute to memory deficit in MDD patients by failure in long-term memory formation.
Introduction
Hippocampus is a well-recognized site involved in the pathological model of major depressive disorder (MDD). Several recent studies reported subfield-level abnormalities of the hippocampus in MDD, suggesting these subfields were unevenly affected by the disorder or distinctively contribute to different aspects of MDD [1-3]. Hippocampus is also well-known for its pivotal function in memory, while memory impairments are also commonly reported in MDD patients [4].Hence, in the current study, we recruited a relatively large sample of MDD patients to test for volumetric alteration in hippocampal subfields and their relationships with memory performance.
Methods
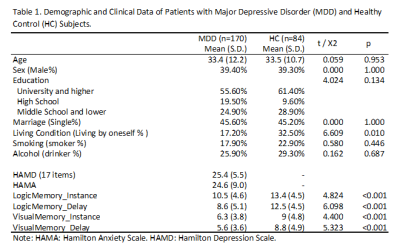
175 MDD DSM-IV criteria diagnosed MDD patients and 84 age, sex-matched healthy control subjects were enrolled in this study (Table 1). Written consents were obtained from all subjects. The severity of depression and anxiety symptoms were assessed using the Hamilton Rating Scale for Depression (HAMD, 17-item) and Hamilton Anxiety Scale (HAMA) respectively. Memory performance was assessed from two aspects – logical and visual. In short, subjects were asked to retell a story right after the story was told by the examiner (logical_instance) and 30 mins after (logical_delay); and draw a picture right after the picture was shown (visual_instance) and 30 mins after (visual_delay).High resolution whole-brain T1 weighted images were obtained using a MPRAG sequence (TR/TE = 1900/2.2ms; inversion time= 900 ms;flip angle = 9°; matrix= 256 × 256; FOV=256 × 256 mm; section thickness, 1 mm) via a Simens 3.0 T scanner. The images were then automatically segmented using FreeSurfer software (V. 6.0) (http://surfer.nmr.mgh.harvard.edu/). Hippocampal subfield segmentation was performed using a module in FreeSurfer that employs a tetrahedral mesh-based probabilistic atlas built from manually delineated hippocampi using in-vivo and ex-vivo data[5]. By this algorithm, the volume of the whole left and right hippocampus and 14 subfields were obtained (Fig 1). All segmentation was visually verified following a quality control protocol that is similar to the ENIGMA protocol (http://enigma.ini.usc.edu/).
We used multivariate analysis of covariance (MANCOVA) to examine volume differences between groups, with age, sex and intracranial volume as covariates. Associations were also examined using partial correlation analysis between volume measurements and clinical/memory ratings with the same covariates.
Results
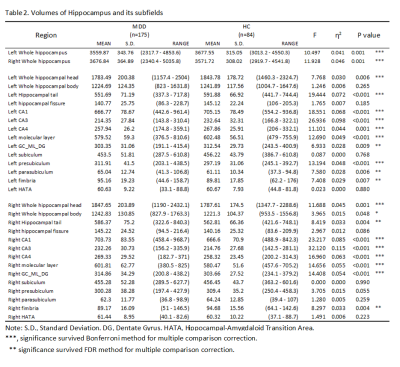
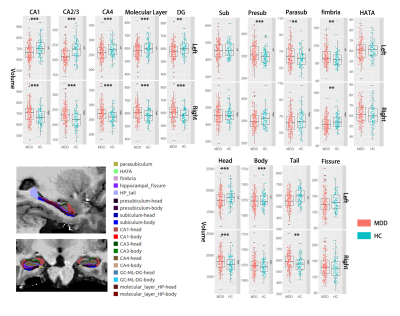
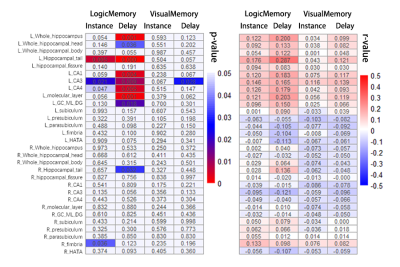
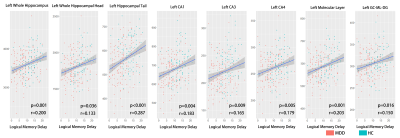
Volumes of the whole hippocampus were significantly smaller in patients with MDD as compared with HC (bilateral, p=0.001). Subfields analyses revealed that smaller volumes in bilateral CA (CA1-4, GC_ML_DG and molecular_layer, all Bonferroni level significant except for left GC_ML_DG). Volumes in left presubiculum (Bonferroni level significant), parasubiculum (FDR level significant) and bilateral fimbria (FDR level significant) were also smaller in patients with MDD relative to HC (Table2, Fig 1). The subregional-level analysis showed that hippocampal head and tail were smaller in patients (bilateral, Bonferroni level significant) but not the hippocampal body.Correlation analyses revealed that volumes in bilateral hippocampal tail were correlated with both HAMD (left: p=0.012, r=0.193; right: p=0.006, r=0.208) and HAMA (left: p=0.004, r=0.218; right: p=0.004, r=0.221) scores. Patients showed poorer memory performance compared with HC (both logical and visual, instance or delay, p<0.001). However, the correlation analyses showed that associations were mostly seen between left hippocampal subfields and logical memory test performance (especially delayed recall) but not visual memory test (Fig 2&3).
Discussion
Consistent with the previous study[2], we found that hippocampal volume was smaller in patients with MDD, especially “core” hippocampal subfields – CA and DG. Although the hippocampal tail was significantly smaller in patients with MDD, we also found that smaller volumes in bilateral hippocampal tail related to milder depression and anxiety symptoms. This seemingly contradictory finding could emphasize the importance of hippocampal tail in MDD and complex relationships between them. For example, Maller et al. reported that larger hippocampal tail predicts remission to anti-depressant medications in MDD patients[1]. Our results could suggest compensatory hypertrophy in the hippocampal tail in patients with severe symptoms.Interestingly, we found volumes of “core” hippocampal subfields – CA and DG relate with logical memory performance but not visual memory performance, and this association showed a strong left-lateralization effect. This lateralized effect could be explained by that the verbal based logical memory test – language is left-lateralized[6]. The correlation effects were stronger in the delayed logical memory test, suggesting hippocampal dysfunction could lead to failure in the transformation from short-term memory to long-term memory.
In conclusion, our study suggests that (1) the “core” hippocampal structures are more involved in MDD than other structures; (2) the hippocampal tail could be important in MDD, but the relationship between them could be complicated; (3) smaller hippocampus could suggest failures in long-term memory formation which could lead to memory deficits in MDD patients.
Acknowledgements
This study was supported by National Nature Science Foundation (Grant NO. 81671669), Science and Technology Project of Sichuan Province (Grant NO. 2017JQ0001).References
1. Molecular psychiatry 23.8 (2018): 1737-1744.
2. Biological psychiatry 85.6 (2019): 487-497.
3. Journal of Magnetic Resonance Imaging 49.6 (2019): 1760-1768.
4. Trends in cognitive sciences 11.2 (2007): 70-76.
5. Neuroimage 141 (2016): 542-555. 6. Journal of Clinical and Experimental Neuropsychology 15.4 (1993): 608-618.
Figures