1988
dACC Dysfunction During Inhibitory But Not Excitatory Motor Control in OCD: Glutamate Dysmodulation Estimated using ¹H fMRS
Jeffrey A. Stanley1, Asadur Chowdury2, Dalal Khatib2, Phillip Easter2, David R. Rosenberg2, and Vaibhav A. Diwadkar2
1Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States, 2Wayne State University School of Medicine, Detroit, MI, United States
1Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States, 2Wayne State University School of Medicine, Detroit, MI, United States
Synopsis
Loss of the E/I balance of the dACC in OCD was assessed using a combination of a) motor control tasks with distinct excitatory and inhibitory modes of responding, and b) ¹H fMRS to quantify evoked glutamate changes. Basal (task-independent) glutamate levels were comparable to healthy controls, as were levels during excitatory modes of responding. However, the inhibitory mode of responding induced a specific deficit in the degree of dACC glutamate modulation in OCD patients. In surmounting shortcomings of fMRI, ¹H fMRS successfully identified loss of excitation in the E/I balance of the dACC during specific phases of motor control.
Background:
Obsessive compulsive disorder (OCD) is a severe, highly prevalent, and chronically disabling disorder characterized by recurrent intrusive, obsessive thoughts and/or repetitive, compulsive behaviors that cause significant distress and impairment in functioning. Network modeling of fMRI signals has documented the intricate role of the dorsal anterior cingulate cortex (dACC) in mediating network interactions associated with top-down cognitive and excitatory motor control1, 2 and identified that this mediation is impaired in OCD3, 4. However, it is unclear if these effects result from impairments in the excitatory or inhibitory contributions of the dACC, both roles of dACC-related control. Brain networks rely heavily on the engagement of glutamatergic and GABAergic neurons that are highly integrated into neural ensembles or microcircuits to establish unique balances between the excitatory and inhibitory (E/I) synaptic drive. This balance serves as the functional basis of coherent networks5, 6, is likely to drive dACC function in health, and may be disrupted in OCD. These shifts in the E/I synaptic balance cannot be characterized by fMRI, but can be characterized in vivo with ¹H fMRS7. Here, using a combination of a specifically developed motor paradigm (with distinct excitatory or inhibitory modes for responding) and ¹H fMRS, we investigated functional imbalances in the E/I synaptic drive of the dACC in OCD youth.Methods:
Data were acquired in OCD patients (6 males and 4 females; mean age, 16.4±2.8 years; age range 12 to 20 years) and 11 healthy individuals (HC; 8 males and 3 females; mean age, 16.5±2.5; age range 12 to 21 years). The scanning protocol was conducted on a 3T Siemens Verio system using a 32-channel volume head-coil. Following the MPRAGE T1-weighted structural images, a single-voxel was prescribed midline in the dACC (2.0x1.7x1.2cm3 or 4.1cm3; Figure 1) using the AVP approach8, followed by B0-field shimming using FASTESTMAP. A variant of visually-guided motor control tasks1, 4, with distinct excitatory and inhibitory modes for responding was carried out during the ¹H fMRS. During the Excitatory mode, participants tapped their right forefinger to flashing green or red probes (50/50% distribution, 100% responses). During the Inhibitory mode, participants responded only to green probes (80% responses) but inhibit responses to red probes (20% responses). Each mode included a .1s probe stimulus duration and six 32s task epochs interspersed with 16s rest epochs. A total of 19 consecutive ¹H MRS measurements were acquired during each mode (PRESS with OVS and VAPOR, TE=23ms, TR=2.67s, 6 averages/measurements, 2048 data points; scan time/mode 5:04min). A control comparison condition (participants fixated on a crosshair, 3:28min) preceded the motor task. ¹H spectra from the six excitation and inhibition epochs and the control condition, were phased (0th and 1st order) and frequency shift corrected prior to averaging and quantification using LCModel (v6.3) (Figure 1). A repeated measure generalized estimating equations (GEE) approach with task condition as the independent variable was used to test significant glutamate changes between Excitation or Inhibition, and the control condition for each group (SAS GENMOD; SAS Institute Inc). Between group comparisons on the % change in glutamate between Excitation or Inhibition relative to the control conditions were also tested using GLM statistics.Results:
Relative to the control condition, increases in dACC glutamate during the Excitation mode were observed in both HC and OCD individuals (χ2=4.49, p=.034 and χ2=6.35, p=.012) (Figure 2). However, groups did not differ from each other (2.1±0.7% vs 3.4±0.9%, χ2=1.07, p=.30). By comparison, the Inhibition mode was highly sensitive in inducing group differences. In this mode, dACC glutamate modulation was significantly higher compared to the control condition in HC (χ2=5.66, p=.017), whereas OCD demonstrated a non-significant decrease in glutamate relative to the control condition (χ2= 1.80, p=.18) (Figure 2). Moreover, the change in glutamate modulation during inhibition was significantly different between groups (2.5±.9% vs -1.4±0.9%, χ2= 7.14, p=.0076). Notably, the “basal” dACC glutamate levels acquired during the fixation crosshair condition did not show a significant group difference (χ2= .007, p=.93), emphasizing the importance of task-based ¹H fMRS in quantifying glutamate differences between OCD and HC.Discussion:
The “basal” E/I steady state of glutamate (fixation crosshair control condition) is similar between OCD and HC, indicating that specific task characteristics evoke pathology. In this framework, inhibitory (but not excitatory) motor control induces a deficit in the functional glutamate response in the dACC. These results are the first assessment, and evidence of impaired task-specific functional neurochemistry of the dACC in OCD. They suggest that inhibitory modes of motor control result in a loss of excitation in the dACC’s E/I balance. That in OCD, the dACC’s E/I balance appears unaffected during excitatory modes of motor control are evidence for a highly task-specific pattern of dysfunction. Further studies (under different task manipulations) are warranted, yet our results provide a compelling approach toward in vivo characterization of the relative contributions of excitatory vs. inhibitory signaling in the pathophysiology of OCD.Acknowledgements
This work was supported by National Institutes of Health grant R01 MH59299 (DRR) and by the Lycaki-Young Funds from the State of Michigan.References
- Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci. 2015;9:309.
- Diwadkar VA, Asemi A, Burgess A, Chowdury A, Bressler SL. Potentiation of motor sub-networks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS One. 2017;12(3):e0172531.
- Diwadkar VA, Burgess A, Hong E, Rix C, Arnold PD, Hanna GL, Rosenberg DR. Dysfunctional Activation and Brain Network Profiles in Youth with Obsessive-Compulsive Disorder: A Focus on the Dorsal Anterior Cingulate during Working Memory. Front Hum Neurosci. 2015;9:149.
- Friedman A, Burgess A, Ramaseshan K, Easter P, Khatib D, Chowdury A, Arnold PD, Hanna GL, Rosenberg DR, Diwadkar VA. Brain network dysfunction in obsessive-compulsive disorder induced by simple uni-manual behavior: The role of the dorsal anterior cingulate cortex. Psychiatry Res Neuroimaging. 2017;260:6-15.
- Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. NeuroImage. 2012;62(2):1040-1050.
- Tatti R, Haley MS, Swanson OK, Tselha T, Maffei A. Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biological psychiatry. 2017;81(10):821-831.
- Stanley JA, Raz N. Functional Magnetic Resonance Spectroscopy: The "New" MRS for Cognitive Neuroscience and Psychiatry Research. Front Psychiatry. 2018;9:76.
- Woodcock EA, Arshad M, Khatib D, Stanley JA. Automated Voxel Placement: A Linux-based Suite of Tools for Accurate and Reliable Single Voxel Coregistration. J Neuroimaging Psychiatry Neurol. 2018;3(1):1-8.
Figures
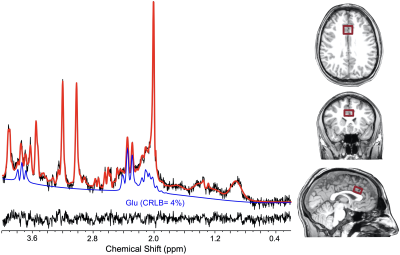
Figure 1: A typical ¹H MRS spectrum from the dACC after combining 6 measurements (6 averages/measurement). The ¹H MRS spectrum in black is superimposed on the LCModel fitted spectrum in red. Below includes the glutamate signal (Glu) in blue and the residual of the fitting in grey. The placement of the single-voxel in the dACC is depicted on the right.
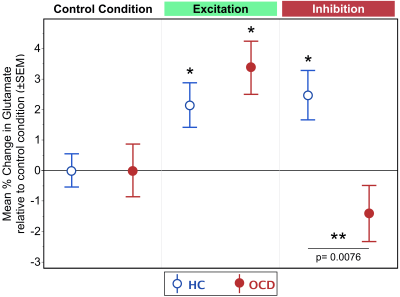
Figure 2: Mean % change in glutamate
relative to the control condition across conditions, fixation crosshair, Excitatory and Inhibition for OCD (red) and HC (blue). Results show no group differences during the Excitatory mode; however, the Inhibitory mode demonstrated an increased dACC glutamate modulation in HC, which was absent in OCD. Errors bars represent ±SEM.