1979
Relative modulations of left DLPFC connectivity with four patho-physologically relevant targets after repetitive TMS in major depression1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Radiology, Cleveland Clinic Lerner College of Medicine, CLEVELAND, OH, United States, 3Neurogolical Institute, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Abnormalities of resting state functional connectivity of several networks have been implicated in the pathology of major depressive disorder. Modulations of functional connectivity of left dorsolateral prefrontal cortex (DLPFC), the site of repetitive transcranial magnetic stimulation (rTMS) for patients inadequately responsive to medication, with four patho-physiologically relevant nodes were studied following rTMS therapy. While the group (N=6) showed improvement in depression following the therapy, only connectivity of left DLPFC with bilateral inferior parietal lobule changed its course – no such change was apparent in connectivity with bilateral anterior cingulate, anterior insula and middle temporal gyrus.
INTRODUCTION
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive, non-convulsive neuromodulation/neurostimulation method that is used in treating major depressive disorder (MDD) for patients who are inadequately responsive to medication treatment. High frequency rTMS of the left dorsolateral prefrontal cortex (lDLPFC) has been shown to be effective in inadequately responsive MDD.1,2 Resting state functional connectivity (fcMRI) of several networks has been reported to be altered in MDD3,4 and application of rTMS has been reported to modulate some of these networks fcMRI.5,6 Understanding the relative modulation/recovery of fcMRI of networks involved in the pathophysiology of MDD and connected with lDLPFC, the site of TMS application, following the therapy could lead to a better understanding of depressive disorder. In this preliminary study, modulation of fcMRI of dorsal/rostral anterior cingulate cortex (ACC), anterior insula (Ins), middle temporal gyrus (MTG) and inferior parietal lobule (IPL), with lDLPFC were studied for a group of MDD subjects with significant improvement following rTMS therapy.METHODS
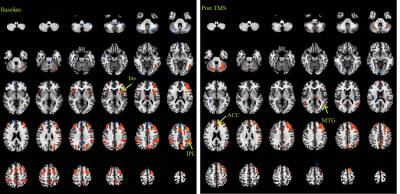
Six MDD patients (52±12 y, 1 male) were scanned at 3T whole body Siemens Prisma scanner (Siemens Healthineers, Erlangen, Germany) at baseline and 6-weeks of rTMS therapy with an institutional review board (IRB) approved protocol. The inclusion criteria required satisfaction of DSM-IV-TR crietria for inadequately responsive MDD to a single antidepressant despite treatment with an adequate dosage for at least 8 weeks with indication for rTMS approved by the Food and Drug Administration (FDA) and Hamilton Depression rating (HAM-D) score of >15). fcMRI data were acquired with a 2D GRE echoplanar scan (TR/TE=2800/29 ms, 31 slices, slice thickness 4mm, no gap, 128×128 matrix, 256mm × 256mm FOV, bandwidth 1954 Hz/pixel, 6/8 partial Fourier, 137 repetitions). Pulse plethysmograph and respiratory bellow were used to monitor physiologic fluctuations. During fcMRI scans a bite bar was used to minimize motion and all subjects were instructed to keep eyes closed. rTMS therapy using a MagPro R-30 magnetic stimulator (MagVenture, Farum, Denmark) consisted of 5 sessions each week (frequency: 10 Hz, power: 120% of the motor threshold (i.e., minimum amount of energy needed to trigger thumb movement), duration of stimulus: 4 s, Inter-train interval: 26 s, number of pulses per train: 75, total number of pulses: 3000.) fcMRI data analysis comprised of: (i) rejection of 1st 4 data-points from timeseries, (ii) physiologic noise correction using RETROICOR,7 (iii) Volume- and slice-wise motion were corrected using SLOMOCO,8 (iv) 2d spatial filtering in Fourier domain, followed by temporal filtering to remove all fluctuations above 0.08 Hz, (v) creating 9 voxel lDLPFC seed based upon maximum correlation with left ACC using InstaCorr routine of AFNI,9 (vi) creating whole brain correlation map with lDLPFC voxel as seed, (vii) converting the correlation to Student’s t, and (viii) generating a whole-brain z-scored connectivity map by normalizing the Student’s t distribution to zero mean and unit variation.10 The seed selection was also guided by the connectivity-based brain atlas (rbmars.dds.nl/CBPatlases.htm). Separate z-scored maps group were generated for baseline and post-rTMS scans and the statistical significance of difference of the maps were determined using 3dtest++ routine of AFNI9 after applying bilateral ACC, Ins, IPL and MTG masks. Because of a-priori significance of the four target regions, significance was decided at P<0.05 level and was also checked at P<0.0125 level.RESULTS and DISCUSSION
HAM-D score following 6-week long rTMS therapy reduced from 20±3 to 11±8 (P<0.03). Z-scored fcMRI correlation maps (P<0.005) with lDLPFC seed at baseline and post TMS therapy are shown in Fig. 1. T-test of difference of the maps showed increased lDLPFC connectivity with bilateral ACC and MTG, and reduced connectivity with bilateral Ins and IPL both at P<0.05 and P<0.0125 levels. Increased lDLPFC-ACC,11,12 lDLPFC-MTG13 and lDLPFC-IPL11 and reduced DLPFC-Ins14 (albeit in the right hemisphere) connectivity has been reported in MDD. Findings from the current study suggest that even though the patients had an overall improvement (lowering in HAM-D), only the frontoparietal network, as determined by lDLPFC-bilateral IPL connectivity showed any recovery. On the other hand, post-TMS lDLPFC-ACC, lDLPFC-MTG and lDLPFC-Ins fcMRI changed in the direction normally associated with worsening of depression. This suggests that frontoparietal connectivity is preferentially affected the by rTMS therapy. It should be noted that this preliminary study did not have an arm sham treatment arm; hence it is still possible that the rate of change of lDLPFC-ACC, lDLPFC-MTG and lDLPFC-Ins fcMRI is slowed down by rTMS, while the frontoparietal fcMRI reverses its course.CONCLUSION
In effective rTMS therapy, the course of frontoparietal (lDLPFC-IPL) fcMRI is preferentially reversed while lDLPFC fcMRI with ACC, MTG and Ins do not exhibit such reversal.Acknowledgements
Cleveland Clinic Research Program Committee partially funded this project.References
1. Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150-2206.
2. George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168(4):356-364.
3. Helm K, Viol K, Weiger TM, Tass PA, Grefkes C, Del Monte D, Schiepek G. Neuronal connectivity in major depressive disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;14:2715-2737.
4. Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330-344.
5. Fan J, Tso IF, Maixner DF, Abagis T, Hernandez-Garcia L, Taylor SF. Segregation of salience network predicts treatment response of depression to repetitive transcranial magnetic stimulation. Neuroimage Clin. 2019;22:101719.
6. Philip NS, Barredo J, Aiken E, Carpenter LL. Neuroimaging Mechanisms of Therapeutic Transcranial Magnetic Stimulation for Major Depressive Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(3):211-222.
7. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162-167.
8. Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014;101:21-34.
9. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173.
10. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119-132.
11. Shen T, Li C, Wang B, Yang WM, Zhang C, Wu Z, Qiu MH, Liu J, Xu YF, Peng DH. Increased cognition connectivity network in major depression disorder: a FMRI study. Psychiatry Investig. 2015;12(2):227-234.
12. Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, Hu D. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74(1):48-54.
13. Ma C, Ding J, Li J, Guo W, Long Z, Liu F, Gao Q, Zeng L, Zhao J, Chen H. Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One. 2012;7(9):e45263.
14. Kandilarova S, Stoyanov D, Kostianev S, Specht K. Altered Resting State Effective Connectivity of Anterior Insula in Depression. Front Psychiatry. 2018;9:83.