1976
Resting-state functional neurobiological signatures associated with psychological distress in early adolescence1University of the Sunshine Coast, Sunshine Coast, Australia
Synopsis
By determining the neuro-functional changes that present with the psychological symptoms of mental illness, potential efficacious, targeted interventions become a possibility. Here we have shown that network dysfunction signatures present in several mental health disorders that underpin emotional regulation and social domain deficits are evident in 12-year-olds with increased psychological distress. Follow-up data from LABS mapping the functional connectome through adolescence and corresponding cognitive and psychological scores will provide valuable insight into the specific neuronal signatures and subsequent divergence pathways that underpin mental health and mental illness.
Background
Adolescence is a critical and dynamic time of neuro- structural and functional changes that can be broadly characterised by reductions in grey matter percentage and synaptic pruning as well as increases in white matter density and volume, myelination, and improved regional network connectivity 1-4. Transition through adolescent development is necessary for establishing and reinforcing efficient structural connections and functional networks. However, it is thought that aberrant neurodevelopment trajectories through this period are associated with the emergence of mental health disorders and first episode onset 5. The increased interest in using neurobiological measures to inform psychiatric nosology has led to a recent shift in the field to describe symptom dimensions in terms of the underlying neuro-circuitry. Recent research has shown resting-state dysfunction between important networks implicated in emotion regulation, cognitive control and self-referential thought in several mental health disorder. However, the emergence of these aberrant functional patterns and temporal relationship with symptom onset is poorly understood. Here we present preliminary findings from the Longitudinal Adolescent Brain Study (LABS) investigating the relationship between psychological distress and their resting-state neuro-functional underpinnings in a general population sample of young people with and without formal diagnoses.Methods
Multimodal neuroimaging was acquired on a 3-Tesla Siemens Skyra scanner with 64-channel coil at the Nola Thompson Centre of Advanced Imaging, SCMNTI. Functional connectivity was analysed from multi-slice resting-state fMRI (rsfMRI), T2* echo-planar BOLD sequences acquired with eyes closed (FOV=240x240mm, matrix size = 80x80, 48 slices, slice thickness = 3mm, TR/TE = 1600/30ms, 300 volumes, multi-slice factor = 4, acceleration factor = 2, scan duration = 9 minutes) acquired with eyes closed. Whole brain, 3D T1-weighted, (MPRAGE) structural MRI acquisitions were used for co-registration (isotropic resolution = 0.9mm, TR/TE/TI = 2200/1.77/850msec, flip angle = 7°, FOV = 230mm, matrix = 256x256, scan duration = 4 minutes). Analysis was carried out in the Matlab-based toolbox CONN 6. In brief this involved: 1: pre-processing of functional and anatomical volumes using SPM8 realignment, outlier identification, co-registration, segmentation, normalization to standard MNI space (DARTEL stream), smoothing (slice-time was omitted as the multi-slice EPI sequence provided sub 2000 TR). 2: Control of residual physiological and motion artefacts - scrubbing, denoising, Global Regression, band-pass filtering of 0.01-0.08 Hz and finally regressing out nuisance signals related to white matter, whole-brain and cerebrospinal fluid signal. Individual 116 x 166 functional connectivity matrices were then generated using the Anatomical Automatic Labelling template and network analyses was carried out using the CONN defined large-scale brain network template. An overview of the data processing pipeline is depicted in Figure 1. Psychological distress scores were measured by the Kessler-10.Partial correlation analysis, accounting for age (months) was carried out to investigate the association between psychological distress score and 1: functional connectivity within and between the salience, frontoparietal and default mode networks. 2: whole brain ROI-to-ROI functional connectivity. All functional network-based statistical analysis was carried out in CONN 6.
Results
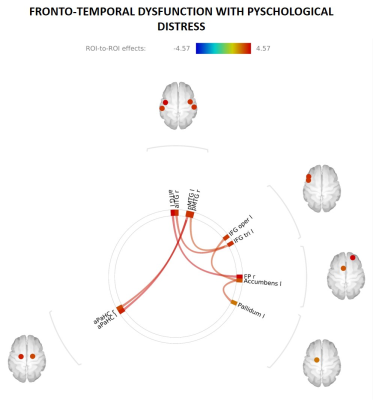
Network dysfunction signatures present in several mental health disorders that underpin emotional regulation and social domain deficits were evident in 12-to-13-year-olds with increased psychological distress. Whole-brain correlation analysis revealed that increased K10 scores were significantly associated with increased connectivity between PFC and the medial and inferior temporal cortices. That is, increased psychological distress in adolescence is associated with disrupted connectivity between regions of the brain that are implicated in higher order executive function, such as top down control of fear. Connectivity in these regions – PFC, medial and inferior temporal cortices – has been shown to be impaired in several mental health disorders 7,8. Significant correlations (surviving FDR correction) between whole-brain connectivity and K10 scores are shown in Figure 2. Functional connectivity analysis within and between the salience, frontoparietal and default mode networks did not survive FDR correction.Conclusion
In summary, we have shown that network dysfunction signatures present in several mental health disorders that underpin emotional regulation and social domain deficits are evident in 12-13-year-olds with increased psychological distress. Follow-up data from LABS mapping the functional connectome through adolescence and corresponding cognitive and psychological scores will provide valuable insight into the specific neuronal signatures and subsequent divergence pathways that underpin mental health and mental illness.Acknowledgements
No acknowledgement found.References
[1] L.P. Spear, Adolescent neurodevelopment, J Adolesc Health, 52 (2013) S7-13.
[2] J.L. Kays, R.A. Hurley, K.H. Taber, The dynamic brain: neuroplasticity and mental health, J Neuropsychiatry Clin Neurosci, 24 (2012) 118-124.
[3] B.J. Casey, R.M. Jones, T.A. Hare, The adolescent brain, Ann N Y Acad Sci, 1124 (2008) 111-126.
[4] B. Casey, R.M. Jones, L.H. Somerville, Braking and Accelerating of the Adolescent Brain, J Res Adolesc, 21 (2011) 21-33.
[5] B. Nelson, P.D. McGorry, M. Wichers, J.T.W. Wigman, J.A. Hartmann, Moving From Static to Dynamic Models of the Onset of Mental Disorder: A ReviewMoving From Static to Dynamic Models of the Onset of Mental DisorderMoving From Static to Dynamic Models of the Onset of Mental Disorder, JAMA Psychiatry, 74 (2017) 528-534.
[6] S. Whitfield-Gabrieli, A. Nieto-Castanon, Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks, Brain Connect, 2 (2012) 125-141.
[7] D.J. Oathes, B. Patenaude, A.F. Schatzberg, A. Etkin, Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging, Biol Psychiatry, 77 (2015) 385-393.
[8] L. Wang, D.F. Hermens, I.B. Hickie, J. Lagopoulos, A systematic review of resting-state functional-MRI studies in major depression, J Affect Disord, 142 (2012) 6-12.
Figures
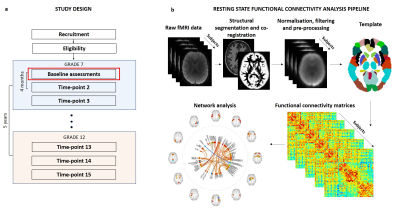
LABS design (a). Participants are recruited at baseline, time-point 1 (Tp1) at 12-years-old in grade 7. Follow-up assessments are carried out every 4 months for 5 years. Each assessment block contains a battery of psychological and cognitive tests, MRI and EEG. Preliminary data presented here is from the first time-point. rsfMRI and structural datasets were processed through the CONN toolbox (b). Individual functional connectivity matrices were then generated using the Anatomical Automatic Labelling or network template.