1975
The longitudinal adolescent brain study: subcortical volume correlates of psychological distress in early adolescence1University of the Sunshine Coast, Sunshine Coast, Australia
Synopsis
Mapping structural trajectories across adolescence provides valuable insights into the “typical” pathway as well as the developmental emergence of mental illness during this dynamic period. Here we present preliminary findings investigating the relationship between the subcortical structures, hippocampus and amygdala (sub-structures known to play important roles in fear pathways and higher order executive functions) and psychological distress measures from the first two time-points in the Longitudinal Adolescent Brain Study. By determining the neuronal changes that present with the psychological symptoms of mental illness, potential efficacious, targeted interventions become a possibility.
Background
Worldwide, one in four people will be affected by a mental or neurological disorder at some point in their lives1. Although poor mental health can affect a person throughout their lifetime, the prevalence of mental health disorders is greatest among 18-24-year-olds 2. More than half of all mental health problems emerge during adolescence, with anxiety, mood, and psychotic disorders being among the most common forms of illness that effect young people 2,3. Consequently, there is now intense interest in large-scale, longitudinal studies that target adolescence for both biomarker detection and intervention in a number of disorders 4. The hippocampus and amygdala have justifiably been the focus of much mental health research 5,6 due to their putative roles in top-down processing control of emotion, fear and anxiety 7-9. However, identification of structural biomarkers of mental illness have been limited as current literature is lacking in the observation of neuro-structural changes preceding first episodes. The Longitudinal Adolescent Brain Study (LABS) is a prospective cohort study being conducted at the Sunshine Coast Mind and Neuroscience Thompson Institute (SCMNTI). Its primary aim is to track longitudinal changes in adolescents to better understand the onset and progression of mental illness in the transition from early adolescence to adulthood. Here we report whole and sub-structural hippocampal and amygdala volume correlates of psychological distress in early adolescence recruited for LABS.Methods
Multimodal neuroimaging was acquired on a 3-Tesla Siemens (Erlangen, Germany) Skyra scanner with 64-channel head and neck coil at the Nola Thompson Centre of Advanced Imaging, SCMNTI. Baseline subcortical hippocampal and amygdala volumes were derived from the whole brain, 3D T1-weighted, (MPRAGE) structural MRI acquisition (isotropic resolution = 0.9mm, TR/TE/TI = 2200/1.77/850msec, flip angle = 7°, FOV = 230mm, matrix = 256x256, scan duration = 3:57minutes). Automated hippocampal subfield and amygdala nuclei segmentation was carried out using the FreeSurfer processing stream 10,11 in 39 participants (12-13 years-old) recruited for LABS who had psychological distress scores measured by the Kessler-10 12. See Figure 1 for study design and analysis pipeline. In a subset of 24 participants the first 4-month follow-up Tp2 was available and was used to carryout longitudinal follow up analysis.Left hippocampal subfield analysis: As this was a preliminary analysis and considering recent literature has reported significant reduction in the Cornu Ammonis (specifically CA1 and CA2-3), subiculum and dentate gyrus in subjects with mental illness (most notably MDD), compared to controls, we limited subfield analysis to these four structures out of the possible 12 subfields segmented by FreeSurfer’s processing stream. Head and body segmentation measures from the FS60 parcellation output 11 were combined to produce total subfield volumes.
Left amygdala nuclei analysis: Previous neuroimaging exploration has shown whole volume differences between cohorts with mental illness and controls 13. However, the amygdala comprises multiple interconnected nuclei with distinct connections and functions. Much of the literature surrounding the mapping and modelling of fear and reward system signalling focuses on the basolateral complex (BLA) and central nucleus (Ce) of the amygdala 14. For this preliminary study we have therefore limited analyses to the basal (BA), accessory basal (AA) and lateral (LA) nuclei that make up the BLA, and the central nuclei (Ce) segmentation volumes.
Results
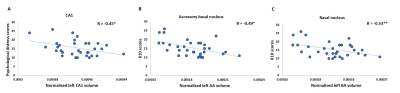
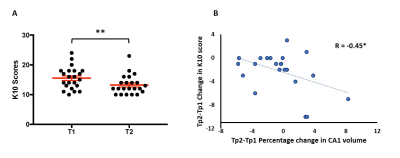
Non parametric partial correlation analyses controlling for sex revealed significant negative association between left whole amygdala volume and psychological distress. Sub-structure analysis revealed that smaller left hippocampal CA1 volume and left basal and accessory basal amygdala nuclei volumes were all significantly associated with higher levels of psychological distress (FDR corrected p=0.028, p=0.028, p=0.006) (Figure 2). Four-month follow-up analysis also revealed an association between change in K10 and CA1 volume (R = -0.45, p = 0.042) suggesting a continued relationship between this hippocampal substructure and psychological distress (Figure 3).Conclusion
Subcortical sub-structures involved within the hippocampal-basolateral amygdala-prefrontal cortex loop, heavily implicated in top-down control of emotion, fear and anxiety, are significantly reduced with increasing levels of psychological distress in 12-13-year-olds, indicating these nuclei and subfields as potential biomarkers of emergence of mental illness. As participants progress through LABS, longitudinal mixed effects modelling of these sub-structure volume trajectories and corresponding changes in functional connectivity between prefrontal cortical regions will provide valuable insight into the divergence pathways that underpin mental health and mental illness. Future work tracking the maturation of subfields and association with disorder onset in a more inclusive sample population is vital and, in this manner, LABS has the potential to provide much needed insight into the divergence pathways of multiple mental health disorders. Here we present the findings recently published in Broadhouse et al., 201915. Recruitment for LABS is ongoing and the continuation of LABS and future analysis of follow-up time-points may reveal stress-sensitive hippocampal and amygdaloid nuclei maturation rates, in particular, the CA1 and BA as potential biomarkers for certain mental health disorders.Acknowledgements
No acknowledgement found.References
1. Levav, I. and W. Rutz, The WHO World Health Report 2001 new understanding--new hope. Isr J Psychiatry Relat Sci, 2002. 39(1): p. 50-6.
2. Ivancic, L.P., B.; Fildes, J.; Perry, Y.; Christensen, H., Youth Mental Health Report, in Mission Australia and Black Dog Institute. 2014.
3. Eggins, P.S., et al., Subcortical volumetric differences between clinical stages of young people with affective and psychotic disorders. Psychiatry Res Neuroimaging, 2018. 271: p. 8-16.
4. Casey, B.J., et al., The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci, 2018. 32: p. 43-54.
5. Blumberg, H.P., et al., Amygdala and Hippocampal Volumes in Adolescents and Adults With Bipolar Disorder. Archives of General Psychiatry, 2003. 60(12): p. 1201-1208.
6. Rosso, I.M., et al., Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry, 2005. 57(1): p. 21-6.
7. Ressler, R.L. and S. Maren, Synaptic encoding of fear memories in the amygdala. Curr Opin Neurobiol, 2019. 54: p. 54-59.
8. Rauch, S.L., L.M. Shin, and C.I. Wright, Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci, 2003. 985: p. 389-410.
9. Kim, M.J., et al., The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res, 2011. 223(2): p. 403-10.
10. Saygin, Z.M., et al., High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage, 2017. 155: p. 370-382.
11. Iglesias, J.E., et al., A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage, 2015. 115: p. 117-37.
12. Kessler, R.C., et al., Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med, 2002. 32(6): p. 959-76.
13. Hamilton, J.P., M. Siemer, and I.H. Gotlib, Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry, 2008. 13(11): p. 993-1000.
14. Janak, P.H. and K.M. Tye, From circuits to behaviour in the amygdala. Nature, 2015. 517(7534): p. 284-92.
15. Broadhouse K.M. · Boyes A. · Winks N. · Dokonal T. · McLoughlin L. · Parker M. · Beaudequin D. · Simcock G. · Lagopoulos J. · Hermens D.F. Subcortical Volume Correlates of Psychological Distress in Early Adolescence. Dev NeuroScience, 2019. DOI:10.1159/000502339
Figures

LABS design (A). Participants are recruited at baseline, time-point 1 (Tp1) at 12-years-old in grade 7. Follow-up assessments are carried out every 4 months for 5 years. MRI analysis was carried out in FreeSurfer (B). 3D surface rendering of amygdala and hippocampus segmentations (tan) and sub-structures are superimposed of the MRPAGE. (C) 3D surface rendering of the sub-structure segmentations (left) along with the 4 defined amygdala nuclei (middle) and hippocampal subfields (right).