1973
The association between stimulant medication, pharmacological MRI, and ADHD symptom severity in the mature and developing brain.1Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 2Department of Child and Adolescent Psychiatry, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 3Department of Child- and Adolescent Psychiatry, GGZ Noord-Holland Noord, Triversum, Alkmaar, Netherlands, 4Brain Plasticity group, Swammerdam Institute for Life Sciences, University of Amsterdam, Amsterdam, Netherlands
Synopsis
We previously used pharmacological MRI (phMRI) to demonstrate that four months of methylphenidate treatment alters dopamine function after washout in medication-naïve children, but not adults, with ADHD. Here, we show in the same children at a 4-5 year follow-up, that the baseline phMRI response in the thalamus and anterior cingulate cortex predicts ADHD severity (hyperactivity scale). However, this association was not moderated by cumulative medication dose. Moreover, no association between phMRI response and symptom severity was found in adults.
Introduction
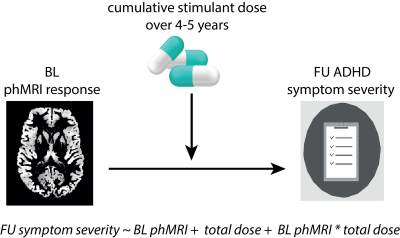
Stimulants such as methylphenidate (MPH) are the main pharmacological treatment for children and adults with attention-deficit hyperactivity disorder (ADHD)1. Using pharmacological MRI, we previously demonstrated in a randomized clinical trial (RCT) that 4 months of treatment with MPH alters dopamine function 1 week after RCT end in children, but not adults with ADHD2. This age-dependent finding is in line with preclinical studies demonstrating long-term effects on the dopaminergic system when MPH treatment occurs during the sensitive period of ongoing brain development, but not when administered in adulthood3. However, in our RCT, changes in dopamine function did not correlate with short-term clinical outcome after 4 months of treatment.PhMRI is based on the principle that neurotransmitter-specific drug challenges evoke hemodynamic responses. Previous studies have validated this approach showing that phMRI can indeed detect relevant changes in the DA system in humans4,5. Here, we assess whether clinical outcome measures at a 4-5 year longitudinal follow-up could be predicted by baseline dopamine function (as measured with pharmacological MRI (phMRI)) and whether this association was moderated by cumulative medication dose.
Methods
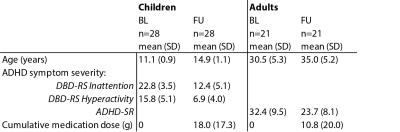
Fifty medication-naïve boys and 49 men with ADHD (DSM-IV, all subtypes) were originally included in a 4-months RCT in which they were randomized to MPH or placebo treatment (1:1). After 4-5 years a naturalistic follow-up was conducted (figure 1, figure 2). We assessed the association between baseline phMRI response (in the striatum, thalamus and anterior cingulate cortex (ACC)), symptom severity and the moderating effect of cumulative stimulant dose (figure 3).Participants underwent two pseudo-continuous arterial spin labeling (pCASL) scans, one before and one 90 min after administration of 0.5 mg/kg MPH (with a maximum dose of 20 mg for children and 40 mg for adults)6. MRI data was acquired on a 3T Philips scanner (Philips Medical Systems, Best, The Netherlands) with an 8-channel receive only head-coil using a pCASL sequence with a 2D gradient-echo echo-planar imaging readout with the following parameters: TR/TE = 4000/14 ms; post-label delay = 1525 ms; label duration = 1650 ms; FOV = 240 × 240 × 119 mm; 75 dynamics; voxel size 3 × 3 × 7 mm, no background suppression, scan time = 10 min. In addition, an anatomical 3D fast field echo (FFE) T1-weighted (T1w) scan was obtained with the following scan parameters: TR/TE = 9.8/4.6 ms; FOV = 256 x 256 x 120 mm ; voxel size = 0.875x0.875x1.2 mm.
Data were processed using the Iris pipeline for cerebral blood flow (CBF) quantification and multi-atlas region segmentation7. To correct for patient motion, the time series were rigidly registered using a group-wise method that uses a similarity metric based on principal component analysis and outlier correction was performed8. All pairs of control and label images were subtracted and CBF was quantified using the single-compartment model9. For each participant, CBF maps were transformed to T1w space and regions of interest (ROIs) were defined using a multi-atlas approach10. For the striatum, thalamus and ACC ROIs, mean CBF values within grey matter were computed. The phMRI response was defined as the CBF change from pre to post MPH administration.
ADHD symptom severity was assessed in children using the Disruptive Behavior Disorder Rating Scale (DBD-RS11) and in adults using the Adult ADHD Self-Report Scale (ADHD-RS12). We conducted a moderation analysis using linear models in R v.3.5.3.
Results
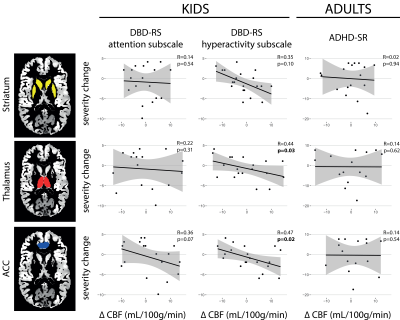
For this ongoing follow-up, 28 children and 21 adults of the original sample were included. There was no difference in ADHD symptom severity between the children and adults that are currently included in the follow-up versus those who are not (children: attention: t(48)=-1.2, p=0.22; hyperactivity: t(48)=-1.0, p=0.22; adults: t(43)=0.01, p=0.99). Symptom severity improved from baseline to follow-up for both the children (attention: t(25)=11.8, p<0.001; hyperactivity: t(25)=10.5, p<0.001) and the adults (t(18)=3.9, p=0.001). Baseline phMRI responses in the thalamus and ACC of children with ADHD were associated with symptom severity (DBD-RS) for the hyperactivity subscale at follow-up (F(1,23)=0.22, p=0.02 and F(1,23)=5.33, p=0.03, respectively). This effect was not moderated by cumulative medication dose (all p>0.05). No associations with the attention subscale in children were found. Moreover, no associations between baseline phMRI response and ADHD symptom severity (ADHD-SR) in adults were found (all p<0.05) (figure 4).Conclusion
These preliminary data suggest that the phMRI response to methylphenidate could be used as a potential predictor of improvement of hyperactivity symptoms in ADHD, at least in medication naïve boys, and irrespective of the use of ADHD medication. Future studies, using larger sample sizes, should replicate this finding.Acknowledgements
We would like to thank all patients and their parents for taking part in the study.References
1. McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong ICK (2012): The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC Pediatr. 12. doi: 10.1186/1471-2431-12-78.
2. Schrantee A, Mutsaerts HJMM, Bouziane C, Tamminga HGH, Bottelier MA, Reneman L (2017): The age-dependent effects of a single-dose methylphenidate challenge on cerebral perfusion in patients with attention-deficit/hyperactivity disorder. NeuroImage Clin. 13: 123–129.
3. Moll GHGH, Hause S, Rüther E, Rothenberger A, Huether G, Al MET, et al. (2001): Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adolesc Psychopharmacol. 11: 15–24.
4. Jenkins BG (2012): Pharmacologic magnetic resonance imaging (phMRI): Imaging drug action in the brain. Neuroimage. 62: 1072–1085.
5. Schrantee A, Václavů L, Heijtel DFR, Caan MWA, Gsell W, Lucassen PJ, et al. (2015): Dopaminergic system dysfunction in recreational dexamphetamine users. Neuropsychopharmacology. 40: 1172–1180.
6. Swanson J, Volkow N (2003): Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 27: 615–621.
7. Bron EE, Steketee RME, Houston GC, Oliver RA, Achterberg HC, Loog M, et al. (2014): Diagnostic classification of arterial spin labeling and structural MRI in presenile early stage dementia. Hum Brain Mapp. 35: 4916–4931.
8. Huizinga W, Poot DHJ, Guyader J-M, Smit H, van Kranenburg M, van Geuns R-JM, et al. (2014): Non-rigid Groupwise Image Registration for Motion Compensation in Quantitative MRI. Biomed Image Regist. (Vol. 8545 LNCS), pp 184–193.
9. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. (2014): Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 73: 102–116.
10. Gousias IS, Rueckert D, Heckemann RA, Dyet LE, Boardman JP, Edwards AD, Hammers A (2008): Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage. 40: 672–684.
11. Pelham Jr. WE, Gnagy EM, Greenslade KE, Milich R (1992): Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 31: 210–218.
12. Kooij S, Boonstra M, Swinkels S, Bekker EM, de Noord I, Buitelaar JK (2008): Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord. 11: 445–58.
Figures