1970
An Integrated PET/MR Study on Functional Connection and Glucose Metabolism of Default Mode Network Nodes for Premature Ejaculation Patients1Nuclear Medicine, Tangdu Hospital of Air Force Medical University, Xi'an, China, 2Radiology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Synopsis
Integrated PET/MR platform was used to observe the functional and metabolic effective connectivity (FC, MEC) patterns between 4 default mode network nodes for premature ejaculation patients, at resting-state and after-ejaculation-state. Significant increased FC between medial prefrontal cortex and posterior cingulate cortex (PCC) and decreased metabolic in PCC were found, after ejaculation. MEC pathways were not the same in different state, but the strength differences of MEC were not significant. There was significant positive correlation between intravaginal ejaculation latency time and the resting-state metabolism in PCC, controlling age factor. These findings gave new thoughts of PE diagnosis and treatment.
Introduction
Premature ejaculation(PE) is the most common sexual dysfunction in adult males, with an incidence up to 30%1. It is believed that central nervous system(CNS) plays a key role in the regulation of ejaculation2. The connection pattern of the default mode network(DMN) is an important means to understand the CNS and diseases3,4. The metabolic abnormalities of the brain were also related to PE. It is necessary to conduct PE studies combining metabolism and function, but there were few relevant studies. The integrated PET/MR platform offers opportunity for simultaneous multimodal study.Methods
The study was approved by institutional board of the Air Force Medical University, China. Ten PE patients (age 30.8±4.9 years) were screened for this study, from patients who visited the department of Urology, Xijing Hospital, Air Force Medical University, and department of Reproductive Men, Northwest Women and Children Hospital, from December 2018 to March 2019, according to the 2014 ISSM diagnostic guidelines and with no history of neurological or psychiatric disease. Intravaginal ejaculation latency time(IELT, 20.5±6.4 seconds) was collected for all PEs. Simultaneous 18F-FDG PET and MRI data(both functional and structural) at resting-state and after-ejaculation-state(after ejaculation, rest for ten minutes before scanning) were collected using a Siemens Biograph mMR integrated PET/MR scanner, in Tangdu hospital. Image data were preprocessed with CONN and SPM12 toolbox. The ROI masks of DMN nodes were provided by CONN, which were bilateral parietal (LP_L/LP_R), medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC). The metabolic effective connectivity (MEC), Funcional connectivity (FC) and standard uptake value ratio (SUVr) of the 4 nodes were calculated using novel method5 combining metabolic and blood oxygen level dependent data. One-sample t-test was performed to test the significance of FC and MEC pathways, and paired t-test was performed to investigate the ejaculation effect for MEC, FC and SUVr (p<0.05, bonferroni corrected for all statistical analysis). The relationship between IELT and SUVr was investigated using spearman partial correlation analysis (controlling variable: age), for the two state.Results
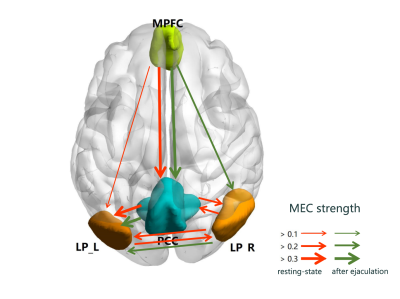
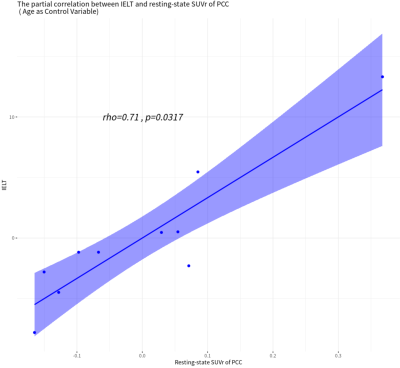
There were always significant fully-connected FC pathways for DMN nodes (p<0.05/6), whether or not ejaculated. The MEC pattern was a bit different (p<0.05/12), pathways from MPFC to PCC, from PCC to LP_L and from LP_R to LP_L remained after ejaculation. But the bidirectional pathway between PCC and LP_R, between bilateral LP, and unidirectional pathway from MPFC to LPL were changed (Fig.1). The ejaculation effect were significant in FC between MPFC and PCC(p=0.006, t=3.58) and the SUVr of PCC(p=0.016,t=-2.97), not the MEC pathways. A significant postive correlation between IELT and resting-state SUVr was observed in PCC (p=0.032, rho=0.71), controlling variable age (Fig.2).Discussion
For the first time, our preliminary study revealed the MEC patterns between 4 nodes of DMN for PE patients, with both before and after ejaculation, using integrated PET/MR. Significant strong unidirectional connection from MPFC to PCC implied that MPFC could mediate PCC function. The PCC metabolism of PE patients showed a significant decrease after ejaculation. PCC’s activity gets lower in task mode6, and the recovery after ejaculation needs a certain time. Sexual arousal and excitement further decreases PCC’s activity7. As the central node of DMN, PCC links to major structures across the whole brain, serves as a hub between higher order cortical system and subordinate brain systems8. Higher resting-state SUVr of PCC related to better performance of PE, i.e., longer IELT. We assumed that higher PCC metabolism represents better information collecting function, which is important for the inhibitory control system to mediate ejaculation procedure. Recent research demonstrated that life-long PE had been suggested to be comparable with impulsive control disorders because of similarities in terms of loss of behavioral control and involvement of the central serotonergic and dopaminergic systems9. Our results showed significantly increased FC strength between MPFC and PCC after ejaculation could be an evidence to this, as prefrontal cortex was thought to be a part of inhibition control system of the brain to assess and controls sexual pleasure and ejaculation impulse from underlying spinal nerves.Conclusion
Although lots remained unclear, our study revealed the specific pattern of the functional and effective connection within DMN for PE patients. More importantly, the disrupted PCC hub function maybe responsible for PE disease. PCC could be a new target for the diagnosis and treatment of PE. Our study has limitations, large sample size and healthy controls should be investigated to ensure the stability of results, especially the MEC results. Besides, the structural alterations of DMN nodes should be researched in the future.Acknowledgements
No acknowledgement found.References
1. El-Hamd MA, Saleh R, Majzoub A. Premature ejaculation: an update on definition and pathophysiology. Asian J. Androl. 2019 Sep-Oct;21(5):425-432.
2. Truitt WA, Coolen LM. "Identification of a potential ejaculation generator in the spinal cord". Science. 2002. 297 (5586): 1566–9.
3. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “Default Mode” Functional Connectivity in Schizophrenia. AJP. 2007;164(3):450-457.
4. Zhao Z, Huang T, Tang C, et al. Altered resting-state intra- and inter- network functional connectivity in patients with persistent somatoform pain disorder. PLoS ONE. 2017; 12(4):e0176494.
5. Riedl V, Utz L, Castrillón G, et al. Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc Natl Acad Sci USA. 2016;113(2):428-433.
6. Buckner RL, DiNicola LM. The Brain’s Default Network: Updated Anatomy, Physiology and Evolving Insights. Nat Rev Neurosci. 2019, 20 (10), 593–608.
7. Zhang B, Lu J, Xia J, et al. Functional insights into aberrant brain responses and integration in patients with lifelong premature ejaculation. Sci Rep. 2017;7(1):1-11.
8. Carhart-Harris, R. L., and Friston, K. J. The default-mode, ego-functions and free-energy: a neurobiological account of Freudian ideas. Brain. 2010. 133,1265–1283.
9. Ozdemir Ö. Is premature ejaculation an impulse control disorder? Medical Hypotheses, 2012, 79(1): 59–62.
Figures