1969
Quantification of cortical thickness changes in bipolar disorder patients following first episode mania: A prospective study1Psychiatry, University of British Columbia, Vancouver, BC, Canada
Synopsis
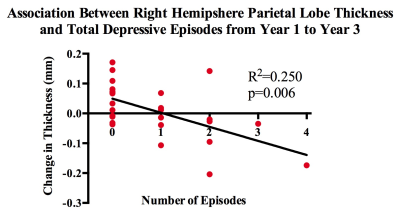
A long disease course of Bipolar I Disorder (BD-I) brings not only with it mood episodes and cognitive impairments, but a progressive change in grey matter morphology. To ascertain how mood episode variables such as type, amount and duration might have an effect on cortical thickness, we conducted a preliminary study in 32 patients from a larger longitudinal study. We found a negative correlation between right parietal lobe thickness and amount of depressive episodes between years 1 and 3 of the disease. These data suggest that early disease course morphological changes may be affected by presence of multiple depressive episodes.
Introduction
Bipolar I Disorder (BD-I) is a leading cause of disability in young persons worldwide1. Not only do patients experience fluctuating mood episodes (manic, depressive and hypomanic), but experience psychosocial and cognitive impairments which persist even during times of symptom remission2–4. In addition to progressive worsening of clinical, functional and cognitive outcomes over the course of illness5, studies have shown progressive brain changes alongside worsening of function6,7. Progressive grey matter loss in frontal regions have been demonstrated8, corresponding with impairments in executive function in BD-I patients. Indeed, episode recurrence has been suggested to be neurotoxic7, thus providing a strong rationale to prevent mood episodes in this clinical population. These findings have highlighted the need to investigate early neurobiological changes in BD-I to better understand, and thereby develop strategies to arrest, neurodegeneration in BD-I. However, very few studies exist which investigate neuroprogressive changes in early stage BD-I, particularly as it relates to episode recurrence. We propose to use MRI data collected from the “Systematic Treatment Optimization Program for Early Mania (STOP-EM)9" project to better understand the longitudinal disease course of BD-I. By comparing the temporal brain morphology information with episode characteristics, we will be able to better understand the relationship between brain structure and episode recurrence in BD-I patients.Methods
Participants: Patients were recruited from the University of British Columbia Hospital and affiliated sties. All patients were diagnosed with BD-I and had their baseline scans within three months of their first manic/mixed episode. Controls were recruited through various forms of advertising.Data acquisition: Imaging was performed on a Philips 3T Achieva scanner in 61 BD-I patients (22.75±4.49 years, 34F) and 44 controls (23±4.01 years, 22F) using a T/R-head coil. Data was collected using a T1-weighted protocol (3D Magnetization Prepared Rapid Gradient Echo (MP-RAGE), TR/TE 7.6/3.5ms, 180 contiguous axial slices, slice thickness=1mm, FOV=25.6cm, flip angle=8°). Patients and controls were scanned at baseline (just following first episode mania (FEM) for patients), at year 1 and at year 3.
Data analysis: All T1 images were bias-corrected using MNIC N310 and skull-stripped using an in-house processing pipeline (SPM8). Corrected scans and their respective brain masks were sent to the Freesurfer v5.1 pipeline for cortical parcellation and subcortical segmentation. All pial and white matter boundaries were inspected and manually corrected. A priori cortical thickness regions of interest (ROIs) were selected based on large-scale bipolar studies11 and recent meta-analyses12. Scans from all patients (n=61) and all controls (n=44) were used for the baseline study. A subset of patients (n=32) were used in the longitudinal study.
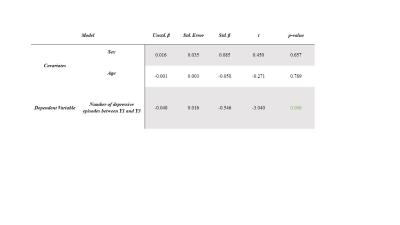
Statistical analysis: At baseline, a two-way independent t-test was used to determine differences in a priori ROI measurements between BD-I patients and healthy controls. A general linear model was used to determine relationships between the change in ROI measurements from baseline to year 1, and subsequently from year 1 to year 3, and episode data (type, amount, and duration). Age and sex were used as covariates in all analyses, and an FDR correction was applied. Mood data was collected using NIH life-charts13.
Results
At baseline, no differences were observed between a priori ROI cortical thicknesses in patients compared with controls. We conducted a preliminary study to probe associations between mood episode variables and change in cortical thickness over repeated time points. We found a strong negative association between number of depressive episodes between years one and three and change in right hemisphere parietal lobe thickness (β=-0.546, p=0.006) (Figures 1&2). However, this finding did not survive FDR correction. No other a priori ROIs were significantly associated with mood episode variables.Discussion
This study is a continuation of previous work where we found greater grey matter volume loss in BD-I patients having had a recurrent mood episode compared to those who stayed well from baseline to year one14. Other studies have shown decreases in cortical thickness and volume in select frontal regions such as the medial prefrontal cortex and anterior cingulate cortex15,16 in patients with BDI. However, these studies are cross sectional and therefore confounded by a longer disease course and an undetermined amount of mood episodes. Abé et al. 2015 found an association between patients having had manic episodes and decrease in DLPFC and interior frontal cortex regions over a 6-year follow up period17. However, they also recruited patients not at FEM and therefore their data could be confounded by episodes prior to this time.Conclusion
In this preliminary analysis, we found that increased number of depressive episodes was associated with reduced right hemisphere parietal lobe thickness in the first three years following first episode of mania. We surprisingly did not find an association between cortical thickness changes and manic episodes, although previous studies have indicated that mania may be particularly neurotoxic7. Small sample size and a low number of recurrent manic episodes in our sample may limit this preliminary analysis. Further analyses in this data set will investigate the impact of medication and substance use on cortical thickness change, as well as potential higher order change trajectories in longitudinal models over the three year follow up.Acknowledgements
We would like to thank the Institutes of Mental Health – Marshall’s Scholars award for grant funding. Additionally, we would like to acknowledge all the participants, research coordinators and MRI technicians at the UBC MRI research center, whom without, this enormous undertaking would not have been possible.References
1. Gore FM, Bloem PJN, Patton GC, et al. Global burden of disease in young people aged 10-24 years: A systematic analysis. Lancet. 2011;377(9783):2093-2102. doi:10.1016/S0140-6736(11)60512-6
2. Zarate CA, Tohen M, Land M, Cavanagh S. Functional impairment and cognition in bipolar disorder. Psychiatr Q. 2000;71(4):309-329. doi:10.1023/A:1004632206684
3. Van Gorp WG, Altshuler L, Theberge DC, Wilkins J, Dixon W. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence: A preliminary study. Arch Gen Psychiatry. 1998;55(1):41-46. doi:10.1001/archpsyc.55.1.41
4. Thompson JM, Gallagher P, Hughes JH, et al. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry. 2005;186(01):32-40. doi:10.1192/bjp.186.1.32
5. Solé B, Jiménez E, Torrent C, et al. Cognitive Impairment in Bipolar Disorder: Treatment and Prevention Strategies. Int J Neuropsychopharmacol. 2017;20(8):670-680. doi:10.1093/ijnp/pyx032
6. Schneider MR, Delbello MP, McNamara RK, Strakowski SM, Adler CM. Neuroprogression in bipolar disorder. Bipolar Disord. 2012;14(4):356-374. doi:10.1111/j.1399-5618.2012.01024.x
7. Barbosa IG, Bauer ME, Machado-Vieira R, Teixeira AL. Cytokines in Bipolar Disorder: Paving the Way for Neuroprogression. Neural Plast. 2014;2014:1-9. doi:10.1155/2014/360481
8. Lim CS, Baldessarini RJ, Vieta E, Yucel M, Bora E, Sim K. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: Review of the evidence. Neurosci Biobehav Rev. 2013;37(3):418-435. doi:10.1016/j.neubiorev.2013.01.003
9. Yatham LN, Uk M, Anna MK, Bond DJ, Lam RW, Torres I. Course and Outcome After the First Manic Episode in Patients With Bipolar Disorder : Prospective 12-Month Data From the Systematic Treatment Optimization Program for Early Mania Projec t. 2009;54(2).
10. Sied JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in mri data. IEEE Trans Med Imaging. 1998;17(1):87-97. doi:10.1109/42.668698
11. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
12. Wang X, Luo Q, Tian F, et al. Brain grey-matter volume alteration in adult patients with bipolar disorder under different conditions: A voxel-based meta-analysis. J Psychiatry Neurosci. 2019;44(2):89-101. doi:10.1503/jpn.180002
13. Koenders MA, Nolen WA, Giltay EJ, Hoencamp E, Spijker AT. The use of the prospective NIMH Life Chart Method as a bipolar mood assessment method in research: A systematic review of different methods, outcome measures and interpretations. J Affect Disord. 2015;175:260-268. doi:10.1016/j.jad.2015.01.005
14. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: A study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519. doi:10.1111/bdi.12437
15. Doris A, Belton E, Ebmeier KP, Glabus MF, Marshall I. Reduction of cingulate gray matter density in poor outcome bipolar illness. Psychiatry Res - Neuroimaging. 2004;130(2):153-159. doi:10.1016/j.pscychresns.2003.09.002
16. Delvecchio G, Ciappolino V, Perlini C, et al. Cingulate abnormalities in bipolar disorder relate to gender and outcome: a voxel-based morphometry study. Eur Arch Psychiatry Clin Neurosci. March 2018:1-8. doi:10.1007/s00406-018-0887-1
17. Abé C, Ekman CJ, Sellgren C, Petrovic P, Ingvar M, Landén M. Manic episodes are related to changes in frontal cortex: A longitudinal neuroimaging study of bipolar disorder 1. Brain. 2015;138(11):3440-3448. doi:10.1093/brain/awv266
Figures