1968
Structural alterations in functional movement disorders: a diffusion weighted imaging study1MNB - HMCS, NINDS - NIH, Bethesda, MD, United States, 2School of Medicine, Stony Brook University, Stony Brook, NY, United States
Synopsis
Functional movement disorders (FMD) patients have an overactive limbic system and an abnormal sense of self-agency. Using diffusion data on a population of 44 (FMD) patients and 44 matched controls, we identified structural anomalies in motor and limbic related areas. ‘Stronger’ (higher FA) limbic white matter reinforces the idea of enhanced limbic effects. Less integrity (higher trace) in areas of the motor system might contribute to the lack of agency.
Introduction
Functional movement disorder (FMD) patients have movements they perceive as involuntary even though they have similar features as voluntary ones. This disorder, also classified as a subtype of conversion disorder under DSM5, is commonly seen in neurology clinics.1 fMRI studies have implicated motor and limbic areas,2-5 however little is known about its neurobiology. Here we report the underpinnings of FMD from structural changes using diffusion MRI.Methods
We analyzed data from a population of 44 FMD patients and 44 age and gender-matched healthy controls (HC) acquired in a 3T Skyra scanner (Siemens) using a 32-channel head coil and a multi-shell diffusion weighted sequence,6 together with T1- and T2-weighted images and anxiety (HAM-A7) and depression (HAM-D8 and BDI9) scores. The diffusion data comprised three b-values (0/300/1100 s/mm2) and 80 volumes (10/10/60) collected with anterior-posterior phase-encoding. Datasets were processed using TORTOISE.6After visual inspection, diffusion images were corrected for Gibbs ringing, motion correction, and eddy-current distortions.10 A non-linear least square tensor fitting was computed for each subject and used to create a group template using a high-performance computer cluster, where we also computed the deformation matrix between each subject space and the template (logJacobian).11
A dual compartment model, accounting for CSF-filled cavities and partial volume contaminations was fitted to compute parenchymal Fractional Anisotropy (FAp), Trace (TRp), and the percentage of parenchymal volume (VF).12 Each scalar map was then transformed to the group template using the computed transformations. We extracted single subject values for the significant clusters (see below) and computed the Pearson correlation between each of them and subjects’ HAM-A, HAM-D and BDI scores.
Statistics:
For each scalar map we performed a t-test in AFNI.13 We used p<0.001 as voxel threshold, with a minimum cluster of 40 voxels. We further computed the effect size of our results (Cohen’s d) by dividing the absolute of the magnitude difference by the pooled standard deviation. Correlations were tested at p<0.05.
Results
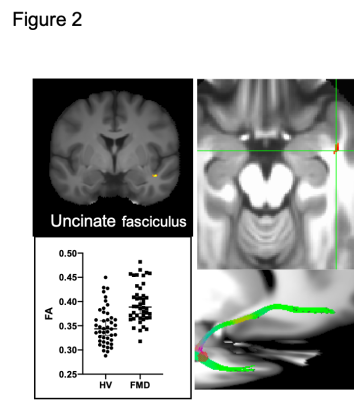
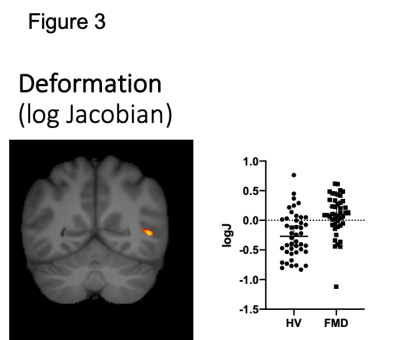
Group differences were observed in all four metrics. FMD patients show deficits in the parenchymal trace of the right precentral cortex and bilateral inferior frontal gyrus (figure 1a) and a larger VF in the right postcentral cortex (Figure 1b). Patients showed increased FAp in the right uncinate fasciculus (figure 2). The deformation matrix indicated group differences in the right superior longitudinal fasciculus (figure 3).All reported results have a large effect size ( d > 0.8). None of the results were driven by anxiety or depression scores.
Discussion
Prior work has shown functional abnormalities in limbic structures and the motor system. The findings here confirm this pattern of abnormality using diffusion imaging. ‘Stronger’ (higher FA) limbic white matter was seen on the right side of the brain, and prior functional fMRI studies show abnormalities of the right amygdala. Less integrity (higher trace) in areas of the motor system might contribute to the lack of agency, the patients’ sense that their movements are involuntary.Conclusion
While this study cannot address whether these changes are cause or consequence of the disorder, it is clear there are neurological changes present in the FMD that correlate with clinical manifestations of the disorder.Acknowledgements
Authors thank Dr LaFaver and Dr Epstein for assistance with patients' evaluations.
This work utilized the computational resources of the NIH HPC Biowulf cluster. (http://hpc.nih.gov), and was supported by the Intramural Research Program of the NIH, NINDS.
References
1. Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, et al. Who is referred to neurology clinics?--the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010; 112(9):747-751.
2. Maurer CW, LaFaver K, Ameli R, Epstein SA, Hallett M, Horovitz SG. Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology 2016; 87(6):564-570.
3. Nahab FB, Kundu P, Maurer C, Shen Q, Hallett M. Impaired sense of agency in functional movement disorders: An fMRI study. PLoS One 2017; 12(4):e0172502.
4. Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC, Jr., et al. Emotional stimuli and motor conversion disorder. Brain 2010; 133(Pt 5):1526-1536.
5. Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord 2011; 26(13):2396-2403.
6. Sarlls JE, Pierpaoli C, Talagala SL, Luh WM. Robust fat suppression at 3T in high-resolution diffusion-weighted single-shot echo-planar imaging of human brain. Magn Reson Med 2011; 66(6):1658-1665.
7. Irfanoglu MO, Sarlls J, Nayak A, Pierpaoli C. Evaluating corrections for Eddy-currents and other EPI distortions in diffusion MRI: methodology and a dataset for benchmarking. Magn Reson Med 2019; 81(4):2774-2787.
8. Irfanoglu MO, Nayak A, Jenkins J, Hutchinson EB, Sadeghi N, Thomas CP, et al. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. Neuroimage 2016; 132:439-454.
9. Pierpaoli C, Jones D. Removing CSF contamination in brain DT-MRIs by using a two-compartment tensor model. . In: International Society for Magnetic Resonance in Medicine. Kyoto, Japan; 2004.
10. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29(3):162-173.
Figures
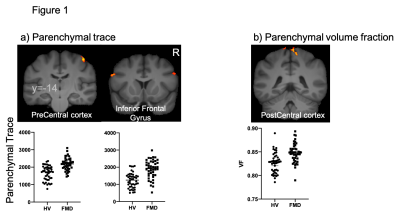
a) Increased parenchymal trace is observed in the right pre central cortex and bilaterally in the inferior frontal cortices of functional movement disorders (FMD) patients.
b) FMD patients show an increased parenchymal volume fraction in the right post central cortex.
These changes might be related with the altered sense of agency observed in FMD patients.