1966
Progressive Microstructural Cortical Changes in Psychotic Spectrum Disorders1Radiology, New York University School of Medicine, New York, NY, United States, 2Department of Rehabilitation Medicine, New York University School of Medicine, New York, NY, United States, 3Psychiatry, New York University School of Medicine, New York, NY, United States
Synopsis
Widespread progressive cortical thinning is a long-standing finding in Psychotic Spectrum Disorders (PSD). While previous histological studies have documented an array of microstructural deficits in the thinning cortex in PSD few techniques have been available to examine these microstructural changes in vivo. We employed the diffusion kurtosis imaging (DKI) technique alongside the classical T1-weighted measure of cortical thickness to describe microstructural changes in the PSD cortex over the first 10 years of the illness. We found, for the first time, that DKI metrics of tissue complexity were significantly positively related to PSD illness progression in the cortex.
Background
A consistent finding in Psychotic Spectrum Disorders (PSD) is widespread cortical thinning that occurs with disease progression1-4. The degree of cortical thinning has been used as a biomarker to predict treatment5, track symptom progression6 and improve patient classification7. Previous histological studies have suggested an array of microstructural deficits may underlie or co-occur with the progressive cortical thinning in PSD and include: increased inflammation, protein accumulation, disruptions in the cell membrane, increased neuronal packing, and decreased dendrite and spine density8-12. However, few techniques have been available to date to examine the microstructural changes in the cortex in PSD in vivo. The aim of this research is to evaluate if the diffusion kurtosis imaging (DKI) technique can describe microstructural changes in the PSD cortex over the first 10 years of the illness. DKI is an extension of DTI that accounts for the non-Gaussian water diffusion contributions to the diffusion MRI signal and provides both mean and directional kurtosis indices that reflect tissue microstructural complexity13. Mapping the DKI-derived measures of microstructural complexity as a function of disease duration may inform upon the biological bases of gray matter thinning pathology in PSD and ultimately contribute to development of targeted treatment approaches.Methods
T1-weighted (T1w) and DKI data was acquired in 26 PSD patients (8 schizophrenia, 11 schizoaffective, 7 bipolar with psychotic features) with an illness duration under 10 years, and 37 healthy comparison controls (HC) (men and women, 18-30 years old). T1w cortical thickness and DKI Mean (MK), axial (AK), an radial (RK) kurtosis metrics were calculated for the 34 bilateral cortical regions of interest (ROI) delineated by the Desikan-Killiany atlas14. Partial Pearson’s correlations, controlling for age, were calculated between illness duration and MRI metrics (thickness, MK, AK, RK). Additional analyses used analysis of covariance (ANCOVA), with age as a covariate, to evaluate differences in MRI metrics (thickness, MK, AK, RK) between chronic patients with an illness duration of 5-10 years (N = 14) and HCs in each ROI. A partial Pearson’s correlation analysis was conducted to assess the relationship between DKI metrics and T1w thickness, controlling for age, across all regions in the cortex and PSD patients examined here. The Benjamini-Hochberg (BH) procedure was employed in each analysis to correct for multiple comparisons and decrease the false discovery rate (FDR)15. Differences were considered significant for q < .05 BH FDR and at trend-level for p < .05.Results
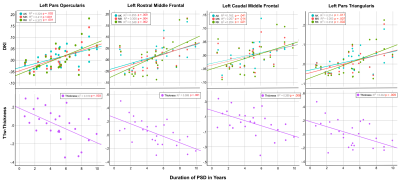
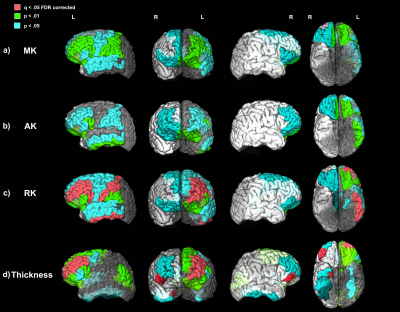
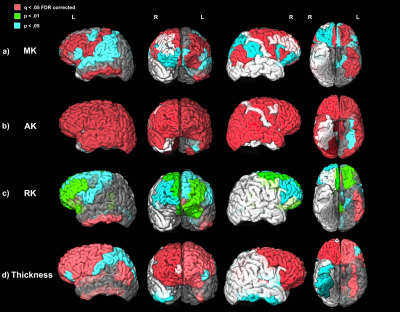
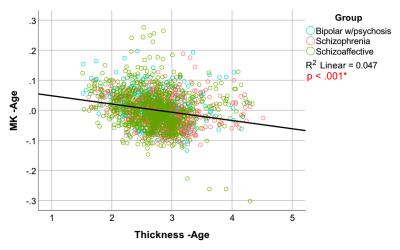
Significant positive relationships between diffusion kurtosis measures and illness duration, and significant negative relationships between T1w thickness and illness duration were found in the patient group in overlapping ROIs located primarily in the frontal and temporal lobes (Figures 1-2). ANCOVA analyses revealed significantly increased AK in 45 out of 68 ROIs tested and significantly decreased cortical thickness in 23 out of 68 ROIs tested in the chronic PSD subgroup (illness duration 5-10 years) compared to HCs (Figure 3). Increased MK and RK were also noted in chronic patients compared with HC for a subset of cortical regions found different by the AK analyses (Figure 3). Partial Pearson’s correlations between DKI metrics and T1w thickness across cortex ROIs, each weighted for volume and controlled for age, revealed significant negative relationships (Figure 4).Discussion
Anatomical T1w MRI studies have documented macroscale GM cortical thinning in widespread ROIs of the frontal and temporal lobe regions in PSD1,3. Results presented here suggest that these changes may be associated with microstructural changes that progress with disease duration. The increased kurtosis documented in PSD may be due to a more restrictive microstructural arrangement (increased microglia and cell packing, increased myelination, iron and protein deposits noted in GM ex vivo) in the cortex that intensifies with disease progression and/or the cortex’s decreased width over-time12,16–18.Conclusion
We demonstrate, for the first time, to the best of our knowledge, that the PSD cortex undergoes progressive microstructural changes with disease duration in the first decade of the disease. In vivo DKI metrics appear to be sensitive to cortical GM microstructural changes related to disease progression and potentially cortical thinning. DKI may provide useful biomarkers of abnormal cortical structure and help elucidate long-standing T1w findings in psychiatric and neurological disorders.Acknowledgements
This work was supported by NIH/NIMH R21 MH085228 and R01 MH108962 awards. We greatly thank all our participants for their help with this study.References
1.Hanford, L. C., Nazarov, A., Hall, G. B. & Sassi, R. B. Cortical thickness in bipolar disorder: A systematic review. Bipolar Disorders (2016).
2.Kong, L. et al. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: A matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Res. - Neuroimaging 231, 176–183 (2015).
3. Gutiérrez-Galve, L. et al. A longitudinal study of cortical changes and their cognitive correlates in patients followed up after first-episode psychosis. Psychol. Med. (2015).
4. Van Haren, N. E. M. et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry (2011).
5.Zhang, W. et al. Discrete patterns of cortical thickness in youth with bipolar disorder differentially predict treatment response to quetiapine but not lithium. Neuropsychopharmacology (2018).
6. Behdinan, T. et al. Neuroimaging predictors of functional outcomes in schizophrenia at baseline and 6-month follow-up. Schizophr Res 169, 69–75 (2015).
7. Clementz, B. A. et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am. J. Psychiatry (2016).
8. Jaaro-Peled, H., Ayhan, Y., Pletnikov, M. V & Sawa, A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophr Bull 36, 301–313 (2010).
9. Bennett, M. R. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol 95, 275–300 (2011).
10.Costa, E. et al. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis 8, 723–742 (2001).
11. Glantz, L. A. & Lewis, D. A. Decreased Dendritic Spine Density on Prefrontal Cortical Pyramidal Neurons in Schizophrenia. Arch. Gen. Psychiatry 57, 65 (2000).
12.Van Kesteren, C. F. M. G. et al. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 7, (2017).
13.Jensen, J. H. & Helpern, J. A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in Biomedicine 23, 698–710 (2010).
14.Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
15.Hochberg, B. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 57, 289–300 (1995).
16.Powchik, P. et al. Postmortem studies in schizophrenia. Schizophr. Bull. (1998).
17. Sokolov, B. P., Tcherepanov, A. A., Haroutunian, V. & Davis, K. L. Levels of mRNAs encoding synaptic vesicle and synaptic plasma membrane proteins in the temporal cortex of elderly schizophrenic patients. Biol. Psychiatry 48, 184–196 (2000).
18.Cho, K. I. K. et al. Microstructural Changes in Higher-Order Nuclei of the Thalamus in Patients With First-Episode Psychosis. Biol. Psychiatry 85, 70–78 (2019).
Figures