1964
Evaluation of white matter neuroinflammation in children with autism spectrum disorder using free-water imaging
Wataru Uchida1,2, Koji Kamagata1, Eiji Kirino3,4, Christina Andica1, Yuya Saito1,2, Akifumi Hagiwara1, Toshiaki Akashi1, Akihiko Wada1, Syo Murata1, Masaaki Hori5, and Shigeki Aoki1
1Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Radiological Sciences, Tokyo Metropolitan University Graduate School of Human Health Sciences, Tokyo, Japan, 3Psychiatry, Juntendo University Graduate School of Medicine, Tokyo, Japan, 4Radiology, Juntendo University Shizuoka Hospital, Shizuoka, Japan, 5Radiology, Toho University Omori Medical Center, Tokyo, Japan
1Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Radiological Sciences, Tokyo Metropolitan University Graduate School of Human Health Sciences, Tokyo, Japan, 3Psychiatry, Juntendo University Graduate School of Medicine, Tokyo, Japan, 4Radiology, Juntendo University Shizuoka Hospital, Shizuoka, Japan, 5Radiology, Toho University Omori Medical Center, Tokyo, Japan
Synopsis
We investigated the differences in white matter pathology between children with autism spectrum disorder (ASD) and typically developing children using single-tensor diffusion tensor imaging (DTI) and bi-tensor free-water (FW) imaging. Tract-based spatial statistics analysis demonstrated significantly increased FW volume fraction in children with ASD compared with that in typically developing children. Increased FW volume fraction may reflect neuroinflammation in children with ASD as a result of inflammatory cytokine accumulation in the white matter. Furthermore, we showed that FW imaging is more sensitive and specific than single-tensor DTI in the evaluation of white matter in children with ASD.
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that usually first manifests in early childhood and is characterized by impaired verbal and nonverbal communication, social deficits, and stereotypical or repetitive behavior.1 Although the recent neuroimmunology research suggests a relationship between ASD in childhood and neural impairment associated with a chronic state of low-grade neuroinflammation, the details of ASD neuropathology remain unclear.2 Single-tensor diffusion tensor imaging (DTI) is useful for evaluating the alterations in various white matter pathways in children with ASD. However, the use of DTI metrics is restricted by the partial volume effect of extracellular free water (FW) in a voxel. Recently, bi-tensor FW imaging has been developed to quantify the contribution of FW and to eliminate the influence of extracellular FW on DTI metrics. Bi-tensor FW imaging may help evaluate the white matter in children with ASD more specifically than DTI using FW-corrected DTI metrics and can be used to measure extracellular FW changes, such as those occurring in neuroinflammation. In this study, we performed a voxel-wise group comparison of bi-tensor FW imaging metrics in children with ASD and in typically developing children using tract-based spatial statistics (TBSS) analysis.METHODS
DTI data of children with ASD and typically developing children were obtained from the Autism Imaging Data Exchange (ABIDE) II3,4: New York University’s Langone Medical Center samples 1 and 2. In total, 30 children with ASD (all male; mean age, 7.95 ± 2.14 years) and age- and sex-matched 18 typically developing children (all male; mean age, 9.68 ± 1.67 years) were enrolled. All DTI data from ABIDE were collected using a 3T magnetic resonance scanner (Allegra; Siemens Healthcare, Erlangen, Germany) and an 8-channel head coil. DTI was acquired at b-values of 0 and 1000 s/mm2 along 64 uniformly distributed directions with spin-echo echo-planar imaging. The imaging parameters used in the New York University samples 1 and 2 were as follows: repetition time, 5200 ms and echo time, 78 ms, with acquisition matrix, 64 × 64; field of view, 192 × 192 mm; slice thickness, 3 mm; number of slices, 50; and acquisition time, 5 min 43 s. Eddy current distortions and motion correction were performed for all DTI data.5 Single-tensor DTI data, such as fractional anisotropy, axial diffusivity, mean diffusivity, and radial diffusivity, were generated using DTIFIT software implemented in FMRIB Software Library 5.0.9 (Oxford Centre for Functional MRI of the Brain, Oxford, UK; www.fmrib.ox.ac.uk/fsl). The maps of bi-tensor FW imaging (FW, FW-corrected fractional anisotropy, FW-corrected axial diffusivity, FW-corrected mean diffusivity, and FW-corrected radial diffusivity) were generated in Matlab (MathWorks, Natick, MA, USA) by fitting the bi-tensor model.6 Then, TBSS7 was applied in FMRIB Software Library 5.0.9 to evaluate the between-group differences. To perform unpaired t-tests, a general linear model was used with the randomise tool from the FMRIB Software Library with 5000 permutations; to make statistical inferences, threshold-free cluster enhancement was performed. A family-wise error-corrected P level < 0.05 was considered significant. The anatomic locations of regions with significant group differences on the WM skeleton were identified from the Johns Hopkins University WM labels atlas.RESULTS
TBSS analysis showed a significantly increased FW volume fraction in the inferior longitudinal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, corticospinal tract, anterior thalamic radiations, and forceps minor in children with ASD compared with that in typically developing children (family-wise error-corrected P < 0.05; Figure1). No significant differences were detected in single-tensor DTI and other bi-tensor FW imaging parameters.DISCUSSION
In this study, we investigated the differences between children with ASD and typically developing children using single-tensor DTI and bi-tensor FW imaging parameters. Significantly increased FW volume fraction was detected in the former, which might reflect neuroinflammation.8 Some recent studies have demonstrated a relationship between ASD and serum levels of high-mobility group box 1 protein, an inflammatory cytokine–like marker with potent proinflammatory activity.9 Our results may provide the evidence for the involvement of inflammatory cytokines in the white matter areas in children with ASD.CONCLUSION
In conclusion, for differentiating between children with ASD and typically developing children, bi-tensor FW imaging exhibits higher sensitivity and specificity than single-tensor DTI. Furthermore, our results demonstrate the presence of neuroinflammation in the white matter areas of children with ASD; this finding may further the understanding of white matter pathology in children with ASD.Acknowledgements
No acknowledgement found.References
1. American Psychiatric Association, DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013. 2. Di Marco B, Bonaccorso CM, Aloisi E, D'Antoni S, Catania MV. Neuro-inflammatory mechanisms in developmental disorders associated with intellectual disability and autism spectrum disorder: A neuro-immune perspective. CNS Neurol Disord Drug Targets. 2016;15(4):448-463. 3. Di Martino A. The autism brain imaging data exchange: towards large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19(6):659-667. 4. Di Martino A, O’Connor D, Chen B, et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data. 2017;4:170010. 5. Mohammadi S, Moller HE, Kugel H, Muller DK, Deppe M. Correcting eddy current and motion effects by affine whole-brain registrations: evaluation of three-dimensional distortions and comparison with slicewise correction. Magn Reson Med. 2010;64:1047-1056. 6. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009; 62(3):717-730. 7. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505. 8. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134(Pt 12):3590-3601. 9. Dipasquale V, Cutrupi MC, Colavita L, Manti S, Cuppari C, Salpietro C. Neuroinflammation in autism spectrum disorders: role of high mobility group box 1 protein. Int J Mol Cell Med. 2017;6(3):148-155.Figures
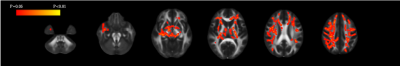
Figure 1. Comparisons of bi-tensor free-water imaging, free-water map
Tract-based spatial statistics showed a significant increase in free water (red–yellow clusters) in the superior longitudinal fasciculus, inferior fronto-occipital fasciculus, corticospinal tract, anterior thalamic radiation, and forceps minor in children with autism spectrum disorder compared with that in typically developing children.