1962
A 3T magnetic resonance study of structural brain alterations in neuroticism1Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 2Institute of Radiology, University Medical Centre Ljubljana, Ljubljana, Slovenia
Synopsis
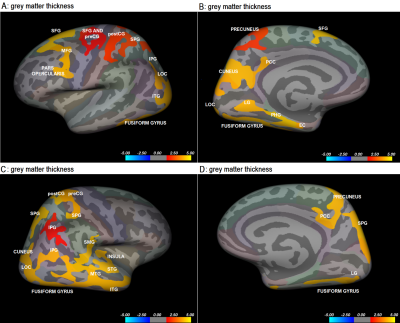
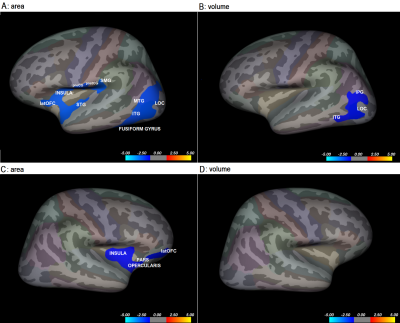
This study investigated the association between alterations in cerebral morphology and neuroticism. Grey matter thickness, cortical area, and volume were calculated in 110 healthy right-handed subjects and correlations with neuroticism scores performed. Negative correlations between neuroticism and cortical area/volume and positive correlations between neuroticism and grey matter thickness were found in occipitotemporal regions. The importance of the ventral visual pathway in emotion processing has been highlighted by structural changes in the occipital and temporal lobe. Alterations in the prefrontal cortex also show the importance emotional control plays in neuroticism. This study provided an additional explanation of structural alterations in neuroticism.
Introduction
Neuroticism is one of the Big Five personality1 traits, and it correlates to the frequency and duration of negative emotions2. Previous research has shown a significant negative association between total brain volume and neuroticism. Individual differences in stress reactivity, vulnerability to life stressors, negative affect, and emotional instability might contribute to reductions in brain volume observed during adulthood3. It is also speculated that high scoring individuals might perceive situations as more stressful than others, and also respond more inadequately to stressors4. Moreover, neuroticism was shown to be correlated with alterations of cortical surfaces4 and cortical thickness5-8. Alterations in volume of different brain regions6,9-14 and changes in cortical folding8,15 have also been demonstrated in individuals with high neuroticism. Our study aims to uncover more specific alterations of cerebral morphology in correlation with the neuroticism score and tries to provide a comprehensive explanation of neural correlates.Methods
Grey matter thickness (GMT), cortical area, and volume were calculated in 110 healthy right-handed subjects (43 females) with a mean age of 43.9 +/- 15.9. High-resolution T1 gradient echo and T2-weighted turbo spin-echo pulse sequences were performed on all participants, and they were tested with Big Five Inventory (BFI) personality test16, which was used for calculating neuroticism score. The following parameters were used in T1-weighted and T2-weighted images, respectfully: TE: 5.9 ms/403 ms, TR: 12 ms/2500 ms, matrix: 320x336/320x336, flip angle: 8°/90°, field of view: 224x235/224x235, voxel dimension: 0.7x0.7x0.7/0.7x0.7x0.7, number of slices in sagittal: 236/236, number of averages: 2/2 and acquisition time: 11 min 55 sec/6min 58 sec. Correlations between GMT, cortical area, volume, and neuroticism were investigated. Cortical reconstruction and volumetric segmentation were performed using Freesurfer image analysis suite 6.0 (Boston, MA, USA). GMT, cortical area, and volume were modeled in Freesurfer's QDEC interface with neuroticism as covariate and age as the nuisance factor. All the maps were smoothed with an FWHM 10 mm kernel. In the analysis, correlations between GMT, volume, cortical area, and neuroticism were explored in all subjects. Automatic algorithm produced regions at the level of statistical significance p<0.05 , afterward correction for multiple comparisons (CMC) was applied in Freesurfer by performing Monte Carlo Simulation at p<0.05.Results
Significant positive association between GMT and neuroticism was found in bilateral fusiform gyri, precunei, cunei, precentral gyri (preCG), postcentral gyri (postCG), superior parietal gyri (SPG), inferior parietal gyri (IPG), posterior cingulate cortices (PCC), lateral occipital cortices (LOC), inferior temporal gyri (ITG), LG, left pars opercularis, medial frontal gyrus (MFG), superior frontal gyrus (SFG), parahippocampal gyrus (PHG), entorhinal cortex (EC) and right insula, supramarginal gyrus (SMG), superior temporal gyrus (STG) and medial temporal gyrus (MTG). The results are represented in Figure 1. On the other hand, significant negative relationship was found between cortical area and neuroticism bilaterally in the insula, lateral orbitofrontal cortex (latOFC) and pars opercularis, left STG, preCG, postCG, SMG, MTG, LOC, and fusiform gyrus. Analysis of correlation between volume and neuroticism yielded negative associations in left IPG, LOC, and ITP and no locations in the right hemisphere (Figure 2).Discussion
Structural changes in the occipital lobe and temporal lobe demonstrate the importance of the ventral visual pathway in processing emotional signals17. These findings suggest that high scoring individuals might perceive situations as more stressful than others. Therefore they are more impacted by stress, which has been shown to be deleterious for brain morphology3,18. Our results support the involvement of the prefrontal cortex in emotional control in neuroticism19. We also demonstrated an essential role of LPC and latOFC in emotion regulation, as shown in the previous studies20.Conclusions
This study corroborated previous findings on structural brain alterations in individuals with high neuroticism but also managed to provide an additional explanation to why the alterations appear in occipital lobe and temporal lobe. Future research could look into the ventral visual pathway and its relationship to neuroticism and possible ways to lessen the negative effects it has on mental health.Acknowledgements
The study was financially supported by The Slovenian Research Agency. Authors declare no conflict of interest.References
1. Digman JM. Personality structure: Emergence of the five-factor model. Annual review of psychology. 1990 Feb;41(1):417-40.
2. Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of personality. 2004 Dec;72(6):1105-32.
3. Bjørnebekk A, Fjell AM, Walhovd KB, Grydeland H, Torgersen S, Westlye LT. Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. Neuroimage. 2013 Jan 15;65:194-208.
4. Knutson B, Momenan R, Rawlings RR, Fong GW, Hommer D. Negative association of neuroticism with brain volume ratio in healthy humans. Biological psychiatry. 2001 Nov 1;50(9):685-90.
5. Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, et al. Neuroanatomical correlates of extraversion and neuroticism. Cerebral cortex. 2006;16(12):1809-19.
6. Blankstein U, Chen JY, Mincic AM, McGrath PA, Davis KD. The complex minds of teenagers: neuroanatomy of personality differs between sexes. Neuropsychologia. 2009;47(2):599-603.
7. Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16(17):1909-12.
8. Riccelli R, Toschi N, Nigro S, Terracciano A, Passamonti L. Surface-based morphometry reveals the neuroanatomical basis of the five-factor model of personality. Soc Cogn Affect Neurosci. 2017;12(4):671-84.
9. DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci. 2010;21(6):820-8.
10. Kong X, Wei D, Li W, Cun L, Xue S, Zhang Q, et al. Neuroticism and extraversion mediate the association between loneliness and the dorsolateral prefrontal cortex. Experimental brain research. 2015;233(1):157-64.
11. Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of aging. 2011;32(12):2162-71.
12. Knutson B, Momenan R, Rawlings RR, Fong GW, Hommer D. Negative association of neuroticism with brain volume ratio in healthy humans. Biol Psychiatry. 2001;50(9):685-90.
13. Kapogiannis D, Sutin A, Davatzikos C, Costa P, Jr., Resnick S. The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Human brain mapping. 2013;34(11):2829-40.
14. Schutter DJ, Koolschijn PC, Peper JS, Crone EA. The cerebellum link to neuroticism: a volumetric MRI association study in healthy volunteers. PloS one. 2012;7(5):e37252.
15. Schultz CC, Warziniak H, Koch K, Schachtzabel C, Güllmar D, Reichenbach JR, Schlösser RG, Sauer H, Wagner G. High levels of neuroticism are associated with decreased cortical folding of the dorsolateral prefrontal cortex. European archives of psychiatry and clinical neuroscience. 2017 Sep 1;267(6):579-84.
16. Avsec A, Sočan G, Vprašalnik BFI. [Big Five Questionnaire]. In A Avsec, editor. Psihodiagnostika osebnosti [Psychodiagnostics of personality]. Ljubljana: Filozofska fakulteta, Oddelek za psihologijo.; 2007. p. 171–178.
17. Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends in cognitive sciences. 2013;17(1):26-49.
18. Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Archives of general psychiatry. 2009 Dec 1;66(12):1373-82.
19. Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31(3-5):373-85.
20. Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR. The Origins of Neuroticism. Perspect Psychol Sci. 2014;9(5):481-96.
Figures