1961
Disrupted integration of single-subject gray matter networks in suicidality of MDD1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2Department of Psychiatry, West China Hospital of Sichuan University, Chengdu, China, 3Psychoradiology Research Unit of Chinese Academy of Medical Sciences (2018RU011), Chengdu, China, 4Department of Nuclear Medicine, West China Hospital of Sichuan University, Chengdu, China, Chengdu, China
Synopsis
In this study, we explore suicidal brain of depression from the level of network connection to capture the complex connectivity alterations of gray matter networks that support higher cognitive and affective processes. We constructed single-subject morphological brain gray matter networks and small-world parameters (Cp, Lp, γ, λ and σ), network efficiency parameters (Eloc and Eglob) and nodal properties (nodal degree, efficiency and centrality). We found decreased σ/Eglob and increased Lp/λ in SU compared to PC and HC. In summary, suicidality involves complex neocortical network organization and showed a weaker integration compared PC and HC.
Background
Most of cerebral gray matter studies investigating the suicidality in depression used voxel-based morphometry analysis. These studies focused on independent brain regions, and could not capture the complex connectivity alterations of gray matter networks that support higher cognitive and affective processes. Now we explore suicidal brain of depression from the level of network connection.Methods
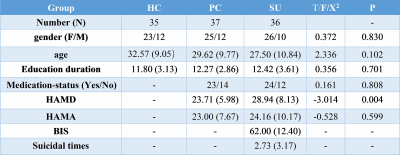
This study was approved by the Ethics Committee of the West China Hospital, Sichuan University, and all subjects provided written informed consent. Participants were included 35 healthy controls (HC), 37 depressions without suicidality (patient controls, PC) and 36 depressions with suicidality patients (SU). Clinical symptoms were assessed with the Hamilton Depression Rating Scale (HAMD), Hamilton Anxiety Rating Scale (HAMA) and Barratt Impulsiveness Scale (BIS). Data analysis was performed as followed steps. 1) Preprocessing: T1 images were normalized to a template space and segmented into gray matter (GM), white matter and cerebrospinal fluid. Then smoothed using a 6-mm fullwidth-at-half-maximum Gaussian kernel. 2) Extraction of brain networks: single-subject morphological brain networks were constructed by estimating interregional similarity in the distribution of regional gray matter volume in terms of the Kullback–Leibler (KL) divergence measure[1]. In detailed, we defined a brain network for each participant comprised of a collection of nodes and edges interconnecting the nodes, in which nodes were brain regions and edges were interregional similarity in the distributions of regional GM volume. 3) Network Analyses: all graph theoretical network analysis in this study was performed using GRETNA. The threshold range was 0.10 < S < 0.40 with an interval of 0.01 to ensure the small-world index was larger than 1. Then area under the curve (AUC) was used to measure across the sparsity parameter S for each network metric. Global network measures included small-world parameters[2] (Cp, Lp, γ, λ and σ) and network efficiency parameters[3] (Eloc and Eglob). Nodal network properties were included the nodal degree, efficiency, and centrality[4]. 4) Statistical analysis: analysis of variance (ANOVA) was used to compare AUC values of each metric among the three groups, followed by post-hoc tests. Statistical significance was set as p < 0.05. We performed correlational analyses between these metrics and the HAMD, HAMA, BIS in patient groups.Results
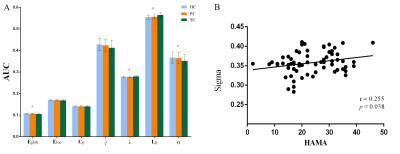
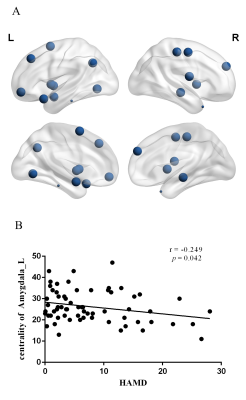
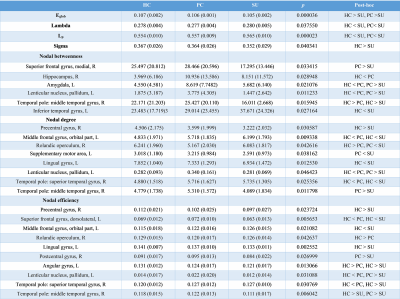
The demographic and clinical data of all participants were showed in Table1. Both depression groups and healthy controls showed a small-world architecture (γ > 1, λ ≈ 1, γ/λ > 1) at all connection densities. SU group had decreased Eglob compared to both PC and HC group, and increased Lp and λ compared both PC and HC group. SU group showed decreased σ than HC group (Table2 and Fig1). Brain regions exhibiting significant differences in nodal betweenness, nodal degree and nodal efficiency were listed in Table2 and Fig2. In MDD patients, we found positive correlation between σ and HAMA scores, negative correlation between nodal betweenness of left amygdala and HAMD scores.Discussion
The human brain network is generally organized as a small-worldness network permitting highly efficient segregated processing (reflected by Cp and Eloc) as well as highly integrated information processing (reflected by Lp and Eglob)[5]. Regularization is defined as increased segregation and/or decreased integration, which is a form of global network disruption[6]. Our finding of decreased global efficiency and increased characteristic path length in SU group showed a weaker integration in the network. This regularization of global properties has been previously reported in MDD by using functional[7, 8] or structural network[9, 10]. The short Lp ensure interregional effective integrity or prompt transfer of information in brain networks, which constitutes the basis of cognitive processes[11]. Thus, the increased Lp may reflect the dysfunction of cognitive process in suicidal patients compared to PC and SU. The Eglob reflects the ability to combine specialized information from distributed brain regions and is mainly associated with long-distance connections[12]. Decreased Eglob suggested a disease-related lowered efficiency of parallel information processing in the brain system of suicidal patients compared with PC and HC. We also found the decreased σ in SU compared to HC. The σ should be calculated to define the small-world network, which is calculated as γ/λ. The decreased σ in SU group result from increased Lp/λ and unchanged Cp/γ. In addition, altered nodal centrality was found in some regions being components of neocortical networks: default mode network (DMN), salience network (SN), control executive network (CEN), sensorimotor network (SEN) and temporal regions. The dysfunction of these networks may be related to reported deficits in emotional processing and impulsivity control function in suicidal brain[13].Conclusions
In summary, the GM connectome of MDD patients with suicidality showed a weaker integration compared PC and HC. Our study shows that suicidality involves complex neocortical network organization and provide insights into the underlying neurobiology of suicidal brain.Acknowledgements
This study was supported by the National Natural Science Foundation (Grant Nos. 81971595, 81771812, 81761128023 and 81621003). Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT16R52) of China, and the Science and Technology Department of Sichuan Province (2018SZ0391) and the Innovation Spark Project of Sichuan University (No. 2019SCUH0003).References
1. Wang, H., X. Jin, Y. Zhang, and J. Wang, Single-subject morphological brain networks: connectivity mapping, topological characterization and test-retest reliability. Brain Behav, 2016. 6(4): e00448.
2. Maslov, S. and K. Sneppen, Specificity and stability in topology of protein networks. Science, 2002. 296(5569): 910-3.
3.Achard, S. and E. Bullmore, Efficiency and cost of economical brain functional networks. PLoS Comput Biol, 2007. 3(2): e17.
4. Rubinov, M. and O. Sporns, Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 2010. 52(3): 1059-69.
5. Bullmore, E. and O. Sporns, The economy of brain network organization. Nat Rev Neurosci, 2012. 13(5): 336-49.
6.Suo, X., D. Lei, L. Li, W. Li, J. Dai, S. Wang, et al., Psychoradiological patterns of small-world properties and a systematic review of connectome studies of patients with 6 major psychiatric disorders. J Psychiatry Neurosci, 2018. 43(5): 170214.
7.Park, C.-h., S.-M. Wang, H.-K. Lee, Y.-S. Kweon, C.T. Lee, K.-T. Kim, et al., Affective state-dependent changes in the brain functional network in major depressive disorder. Social cognitive and affective neuroscience, 2014. 9(9): 1404-1412.
8. Meng, C., F. Brandl, M. Tahmasian, J. Shao, A. Manoliu, M. Scherr, et al., Aberrant topology of striatum’s connectivity is associated with the number of episodes in depression. Brain, 2013. 137(2): 598-609.
9. Mak, E., S.J. Colloby, A. Thomas, and J.T. O'Brien, The segregated connectome of late-life depression: a combined cortical thickness and structural covariance analysis. Neurobiology of aging, 2016. 48: 212-221.
10. Chen, V.C.-H., C.-Y. Shen, S.H.-Y. Liang, Z.-H. Li, Y.-S. Tyan, Y.-T. Liao, et al., Assessment of abnormal brain structures and networks in major depressive disorder using morphometric and connectome analyses. Journal of Affective Disorders, 2016. 205: 103-111.
11.Sporns, O. and J.D. Zwi, The small world of the cerebral cortex. Neuroinformatics, 2004. 2(2): 145-62.
12. Latora, V. and M. Marchiori, Efficient Behavior of Small-World Networks. Physical Review Letters, 2001. 87(19): 198701.
13. Cao, J., X. Chen, J. Chen, M. Ai, Y. Gan, W. Wang, et al., Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J Affect Disord, 2016. 205: 252-263.
Figures