1960
Improving T2 Sensitivity of Magnetic Resonance Fingerprinting for Simultaneous Quantification of Gadolinium and 17O-Water Concentration1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Department of Radiology, University of Michigan, Ann Arbor, MI, United States, 4Department of Neurology, Case Western Reserve University, Cleveland, OH, United States, 5Cleveland Clinic Pre-Clinical Magnetic Resonance Imaging Center, Cleveland Clinic Foundation, Cleveland, OH, United States, 6Department of Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Simultaneous quantification of intravenously injected Gadolinium (Gd) and 17O-water through T1 and T2 shortening effect enables evaluation of BBB permeability to large and small molecules under pathological conditions. Magnetic resonance fingerprinting allows fast and simultaneous T1 and T2 mapping, though improvement in T2 sensitivity is required for accurate quantification of 17O-water in brain. This study demonstrates that the combination of T2-preparation module and small flip angle improves the accuracy in T2 estimation in mouse brain. Further, the feasibility of using MRF method to quantify Gd and 17O-water concentration was explored in a phantom study.
Introduction
The blood-brain-barrier (BBB) plays an important role in maintaining cerebral molecular and water content. Changes in BBB permeability is indicative of BBB disruption under various pathological conditions. Gadolinium (Gd) based contrast agent has been used to evaluate BBB integrity. However, it is only sensitive to severe BBB disruption because of the large molecular size of Gd-based contrast agent. Alternatively, the T2-shortening effect of 17O-water allows the quantification of BBB permeability to water, which can be more sensitive to early-stage BBB disruption.1 More importantly, fast and simultaneous T1 and T2 mapping enabled by magnetic resonance fingerprinting (MRF) provides a unique opportunity for comprehensive evaluation of BBB permeability to both small (water) and large (Gd) molecules. However, the relatively low r2 relaxivity of 17O-water and the short T2 values in brain tissue demands high accuracy and precision in T2 mapping. The aims of this study were: (1) to incorporate T2-preparation modules in MRF sequence for simultaneous T1 and T2 mapping in small animals with improved T2 sensitivity2; (2) to evaluate the feasibility of using MRF for simultaneous quantification of Gd and 17O-water concentrations in a phantom study.Methods
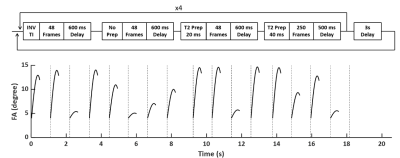
T2-Prepared MRF Sequence: The sequence diagram and flip angle (FA) patterns of the T2-prepared MRF (T2-MRF) sequence are shown in Fig. 1. The sequence consisted of 16 segments with 4 segments preceded by an inversion pulse and 8 segments preceded by T2-preparation module with 20 or 40 ms mixing time. In each segment, FA ramped up sinusoidally from 4° to a maximal value between 6° to 15°. A 600-ms delay was applied between each segment for improved SNR. Constant TR (10 ms) and TE (1.7 ms) were used and 4π dephasing was applied along the slice-selection direction.In Vivo Study: In Vivo studies were performed at 7T on C57/BL6 mice (n=2). T1 and T2 maps were acquired using the T2-prepared MRF sequence. As a comparison, T1 and T2 maps were also acquired using an MRF sequence without T2 preparation3. MRF acquisition used a uniform density spiral trajectory with 48 interleaves to fully sample the k-space with an FOV of 20x20 mm2 and a matrix size of 128x128, yielding a nominal resolution of 156x156 μm2. T1 and T2 maps acquired with saturation recovery Look-Locker (SRLL) and spin-echo (SE) sequences were used as the standards.
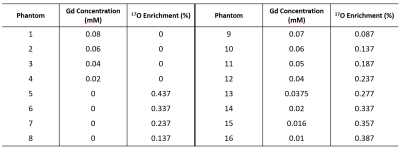
Phantom Study: The phantom consisted of 16 compartments with varied Gd and 17O-water concentration. Compartments 1-8 contained only Gd or 17O-water and were used for calibration of Gd and 17O-water relaxivity. Compartments 9-16 contained mixtures of Gd and 17O-water at various concentrations. MRF sequence without T2-preparation was applied for quantification of T1 and T2. The concentration of Gd and 17O water were determined using the multiple contrast agent model proposed by Anderson et al4:
$$ 1/T_1 = 1/T_{1,0} + r_{1,Gd} [Gd] + r_{1,{H_2}^{17}O} [{H_2}^{17}O] $$ $$ 1/T_2 = 1/T_{2,0} + r_{2,Gd} [Gd] + r_{2,{H_2}^{17}O} [{H_2}^{17}O] $$
where $$$r_{1,Gd}$$$, $$$r_{2,Gd}$$$, $$$r_{1,H_2^{17}O}$$$, and $$$r_{2,H_2^{17}O}$$$ are the relaxivity, and $$$[Gd]$$$ and $$$[H_2^{17}O]$$$ are the concentrations of Gd and 17O-water, respectively. $$$T_1$$$, $$$T_2$$$, $$$T_{1,0}$$$and $$$T_{2,0}$$$ are the relaxation rates with and without contrast agent, respectively.
Results
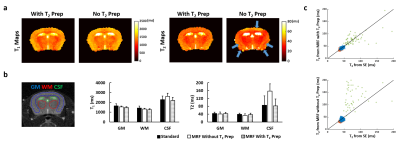
T2 Mapping Accuracy by MRF: While T1 mapping by both MRF methods showed good agreement with the standards, T2-MRF enabled T2 mapping with improved accuracy (Fig. 2a&b). The T2 values were less affected by the strong off-resonance effect in the area close to ear canal and close to the top of cortex using T2-MRF (Fig. 2a). Pixel-by-pixel comparisons between MRF-measured T2 values and the standards are shown in Fig. 2c. T2-MRF showed better agreement with the standards in all three ROIs. T2 measurements in CSF showed larger variations comparing to gray and white matter, likely due to the short mixing time used in the current sequence.Dual-Contrast Quantification: Fig. 3a shows the results of relaxivity calibration for Gd and 17O-water using MRF. Gd showed potent shortening effect for both T1 and T2, while 17O-water only induced T2 shortening but not T1. MRF-estimated concentrations of Gd and 17O-water in the mixed solution (compartments 9-16) are shown in Fig. 3b. Despite significant inhomogeneity, the mean values of the estimated concentrations showed good agreement with the ground truth.
Discussion and Conclusion
This study demonstrates that T2-prepared MRF sequence improves the accuracy of T2 estimation and reduces the artifacts in T2 mapping. Simultaneous T1 and T2 mapping enable by MRF provides the opportunity for simultaneous quantification of Gd and 17O-water concentrations. With improved T2 sensitivity by T2-MRF, further improvement in measurement accuracy can be expected.Acknowledgements
This work was supported by a grant from the U.S. National Institute of Health (R01 EB23704).References
1. Igarashi H, Tsujita M, Kwee IL, Nakada T. Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. Neuroreport. 2014;25:39–43.
2. Hamilton JI, Jiang Y, Chen Y, Ma D, Lo W-C, Griswold M, Seiberlich N. MR fingerprinting for rapid quantification of myocardial T1 , T2 , and proton spin density. Magn Reson Med. 2017;77:1446–1458.
3. Gu Y, Wang CY, Anderson CE, Liu Y, Hu H, Johansen ML, Ma D, Jiang Y, Ramos-Estebanez C, Brady-Kalnay S, Griswold MA, Flask CA, Yu X. Fast magnetic resonance fingerprinting for dynamic contrast-enhanced studies in mice. Magn Reson Med. 2018;2681–2690.
4. Anderson CE, Donnola SB, Jiang Y, Batesole J, Darrah R, Drumm ML, Brady-Kalnay SM, Steinmetz NF, Yu X, Griswold MA, Flask CA. Dual Contrast - Magnetic Resonance Fingerprinting (DC-MRF): A Platform for Simultaneous Quantification of Multiple MRI Contrast Agents. Sci Rep. 2017;7:8431.
Figures