1959
Characterization of kinetic isotope effects and label loss in deuterium-based isotopic labeling studies.1Dept. of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Dept. of Psychiatry, Yale University, New Haven, CT, United States
Synopsis
Deuterium metabolic imaging (DMI) is a novel, non-invasive method to map metabolism from deuterated substrates in 3D. The replacement of protons with deuterons could potentially lead to kinetic isotope effects (KIEs), and loss of deuterons. Knowledge of the KIE levels, and label losses is required for DMI-based measurements of absolute metabolic rates. Here the deuterium KIE and label loss is investigated for glucose and acetate in rat brain in vivo using a double substrate/double labeling strategy. Significant, but predictable and reproducible label losses were observed in the metabolic products lactate, glutamate and glutamine. The measured KIE was relatively small (4-6%).
Introduction
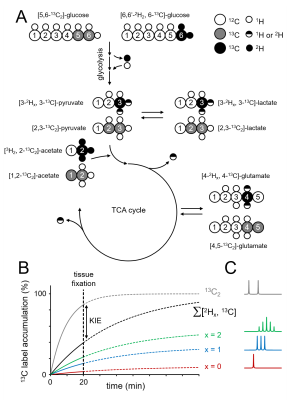
The use of the hydrogen isotope deuterium (2H) as an NMR-detectable label has seen a recent increase with the development of deuterium metabolic spectroscopy (DMS) and imaging (DMI) methods (1–4). Deuterium-based MR methods are characterized by high sensitivity, simple acquisition methods, and relative immunity to magnetic field inhomogeneity, making these techniques very robust. Any isotope labeling strategy can be affected by the presence of a kinetic isotope effect (KIE) by which the rate of a chemical reaction is decreased when one or more atoms of a reactant is replaced with a heavier isotope. In addition to the KIE, 2H labeling approaches can also be affected by 2H label loss during metabolic steps that involve rapid hydrogen exchange. Here we present a study on the KIE and label loss of [6,6-2H2]-glucose, and [2H3]-acetate, two substrates used in DMS and DMI (1,2). Because a robust differentiation between KIE and 2H label loss is not possible with 1H or 2H NMR methods, we employed 13C NMR in combination with 13C and 2H double-labeled glucose or acetate. The 13C chemical shifts and scalar coupling patterns provide a unique and unambiguous spectral fingerprint from which 2H label loss and the KIE for multiple metabolic products, including lactate, glutamate and glutamine can be determined.Methods
All experiments were performed on a Bruker Avance spectrometer (Bruker Instruments, Billerica, MA) operating at 500.13 MHz for 1H and equipped with a 5-mm broadband probe incorporating a single-axis (Z) gradient coil. Direct 13C-[1H] NMR spectra were acquired with a pulse-acquire method (TR=20 s) as 16,384 complex points over a 25.2 kHz (or 200 ppm) spectral width in the presence of adiabatic broadband proton decoupling. Fischer 344 rats were anaesthetized with 1–3% isoflurane in 70%/30% N2O/O2 via a nose cone after which glucose or acetate was administered intravenously. Glucose was administered for 20 min as an equimolar solution of [6,6-2H2,6-13C]-glucose and [5,6-13C2]-glucose (Fig. 1). Acetate was administered for 20 min as an equimolar solution of [2H3,2-13C]-acetate and [1,2-13C2]-acetate. At the end of the infusion animals were euthanized using focused beam microwave fixation, which instantly stops metabolism and prevents autolysis during tissue processing (5). Metabolite extraction and sample preparation were performed using a bead mill and methanol/HCL extraction buffers as previously described (6). 2H label loss was evaluated from the unique spectral patterns of non, single and double-deuterated metabolic products (lactate, glutamate, glutamine) generated from the deuterated substrates (glucose, acetate). The KIE was evaluated by comparing the total amounts of product originating from the deuterated substrate to that originating from the accompanying non-deuterated substrate, whereby the 13C-13C scalar coupling provided a unique spectral feature that allowed unambiguous identification and separation. At 20 minutes after infusion 13C-labeling in downstream metabolites has not yet reached steady state. Therefore, lower levels of 13C-labeling in products originating from substrates that are both 13C and 2H-labeled compared to the products from substrates that are only 13C-labeled can be attributed to a slower metabolic rate induced by the presence of 2H, i.e. a KIE (see Fig.1B).Results
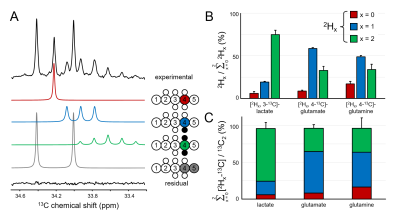
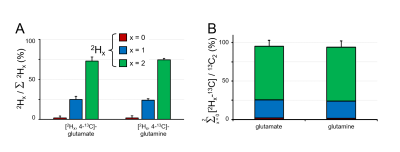
Figure 2A shows the C4 position of glutamate in rat brain extract following the administration of an equimolar solution of [6,6-2H2,6-13C]-glucose and [5,6-13C2]-glucose. The chemical shifts and scalar coupling patterns indicate the presence of four distinct glutamate species. Fig. 2B provides a quantitative summary of the different [2Hx,3-13C]-lactate, [2Hx,4-13C]-glutamate and [2Hx,4-13C]-glutamine species as formed from [6,6-2H2,6-13C]-glucose in rat brain. In DMS and DMI studies employing direct 2H detection, the 2H label distributions of Fig. 2B would lead to 84.3±2.6%, 62.1±1.1% and 58.5±5.2% signal intensity for lactate, glutamate and glutamine relative to the theoretical signal in the absence of label loss. Fig. 2C shows that the sum of lactate, glutamate and glutamine produced from deuterated glucose represents 96.0±12.9%, 96.6±6.2% and 98.0±19.3% of the respective products produced from non-deuterated glucose, indicating a KIE of 4 % or less. Figure 3A summarizes the 2H label loss for glutamate and glutamine in rat brain following the administration of [2H3,2-13C]-acetate and [1,2-13C2]-acetate. For direct 2H-detected DMS and DMI studies, this 2H label loss distribution would translate into 85.6±3.4% and 86.4±2.2% glutamate and glutamine signal intensity, respectively, relative to the hypothetical signal in the absence of label loss. Fig. 3B shows that the sum of glutamate and glutamine produced from deuterated acetate represents 95.5±9.6% and 94.1±8.3% of the respective products produced from non-deuterated acetate, indicating a KIE of 6 % or less.Conclusions
We have demonstrated a small 2H KIE and significant, but reproducible deuterium label loss in rat brain in vivo during metabolism of deuterated glucose and acetate. The 2H label loss from lactate to glutamate is close to statistical expectation, whereas the label loss between glutamate and glutamine conversion appears insignificant. These data are essential for metabolic modeling of dynamic DMI and DMS studies aimed at obtaining absolute metabolic rates.Acknowledgements
This research was supported by NIH grant R01- EB025840.References
1. Lu, Ming, Zhu, Xiao-Hong, Zhang, Yi, Mateescu, Gheorghe, Chen, Wei. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 2017;37:3518–3530 doi: 10.1177/0271678X17706444.
2. De Feyter HM, Behar KL, Corbin ZA, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018;4:eaat7314 doi: 10.1126/sciadv.aat7314.
3. Veltien A, van Asten J, Peeters TH, et al. Glucose metabolism in the brain of an Alzheimer mouse model by deuterium MRS. In: Proc. Intl. Soc. Mag. Reson. Med. 27 (2019). Montreal; 2019.
4. Kreis F, Wright A, Fala M, Hu D, Brindle K. Monitoring treatment response in a murine lymphoma with Deuterium Metabolic Imaging and Spectroscopy. In: Proc. Intl. Soc. Mag. Reson. Med. 27 (2019). Montreal; 2019.
5. De Graaf RA, Chowdhury GMI, Brown PB, Rothman DL, Behar KL. In situ 3D magnetic resonance metabolic imaging of microwave-irradiated rodent brain: a new tool for metabolomics research. J. Neurochem. 2009;109:494–501 doi: 10.1111/j.1471-4159.2009.05967.x.
6. De Feyter HM, Behar KL, Rao JU, et al. A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro-Oncol. 2016;18:1079–1087 doi: 10.1093/neuonc/now088.
Figures