1957
Training intensity induced differential functional networks in rodent1Queensland Brain Institute, The University of Queensland, St Lucia, Australia, 2Centre for Advance Imaging, The University of Queensland, St Lucia, Australia, 3School of Biomedical Sciences, The University of Queensland, St Lucia, Australia
Synopsis
· After spatial learning, large-scale plasticity in functional networks were detected in mice using resting-state fMRI.
· Different training intensity recruited different functional networks.
· Functional connectivity reorganized to be less hippocampus dependent after 1 week of consolidation.
Introduction
Increasing evidence indicates that learning-induced plasticity in functional connectivity is detectable by resting-state fMRI in different species1–3. Memory encoding and consolidation have been suggested to involve multiple brain areas and complex processes4–6. It has been shown that different brain areas are recruited in different kinds of learning tasks. For example, hippocampal, prefrontal cortex and retrosplenial cortex are involved in spatial memory7,8, while amygdala and hippocampus are strongly involved in fear memory9,10 . However, less is known about whether different training paradigms of same task, such as intensive or gradual learning, invoke same or different functional circuits during memory encoding or consolidation. To address this question, we examined the dynamics of functional networks after two paradigms of the same spatial learning, active place avoidance (APA), task where animals need to learn to avoid a shock zone based on spatial cues.Methods
The study was approved by the animal ethic committee of the University of Queensland. Adult C57BL6/J mice at age of 10-12 weeks were trained with 1-Day or 5-Day APA task. In the 1-Day APA, animals received 5 sessions of training within one day (10min per session, inter-session interval=1h). In the 5-Day APA, animals received 1 session of training per day for five consecutive days (10min per session). In the control group, animals were kept in home cage. Animals went through two sessions of fMRI scans at one day and eight days after the training. Another session APA task (probe test) was performed after the second fMRI scan to examine memory retention. MRI was conducted on Bruker 9.4 T system with mice sedated with 0.1 mg/kg/h continuous infusion of medetomidine and 0.5-0.25% isoflurane. The resting-state fMRI was acquired using multiband EPI11 with TR/TE = 300/15 ms, thickness = 0.5 mm, gap = 0.1 mm, 16 axial slices covering the whole cerebrum with in-plane resolution of 0.3 × 0.3 mm2. 2000 volumes were acquired in 10 min and repeated 3 times (total 6000 volumes). Raw data were first pre-processed to reduce motion, noise and nuisance and then registered to AMBMC mouse brain atlas. Seed-based correlation analysis was used to measure functional connectivity across the brain with the mean time-series signal of each brain region extracted as the seed signal for construction of correlation matrix. Two-sample t-test was used to analyse the difference between APA groups and control group.Results
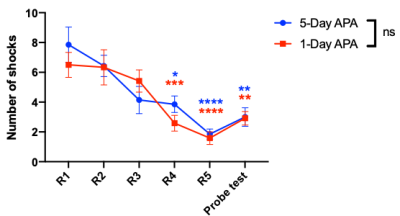
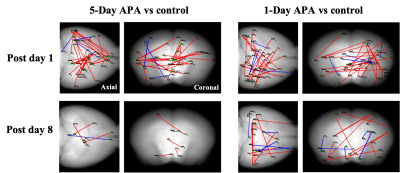
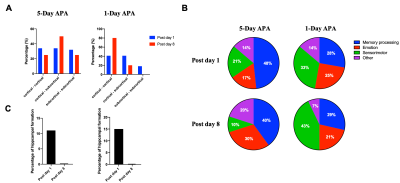
Fig.1 shows the learning curves of mice in both 1-Day and 5-Day APA paradigms. Note that number of shocks decreased significantly in similar manner in these two paradigms of training. In the probe test, all animals still received significantly less shocks compare to the start of the training, indicating long-term memory retention. No significant difference was found between the two training paradigms, showing good and comparable learning. Both 1-Day and 5-Day training altered functional connectivity with the number of changed functional connections decreased from post day 1 to post day 8 in both paradigms (Fig.2). Despite similar behavior, these two paradigms invoked different networks. In 5-Day APA, there are higher percentage of cortical-subcortical connections, while in 1-Day APA, higher percentage of cortical-cortical connections was found after 1 week of consolidation (Fig. 3A). Based on the main functions, we categorized the involved brain areas into 4 groups: memory processing, emotion, sensorimotor and other. Higher percentage of memory processing-related brain areas was found in 5-Day APA compared to 1-Day APA at both time points, whereas 1-Day APA invoked more sensorimotor networks even 1 week after (Fig. 3B). Both paradigms also invoked a large proportion of emotion-related areas. In addition, we also calculated the percentage of brain regions belonging to hippocampal formation. Interestingly, though more than 10% brain regions were hippocampal formation at post day 1 in both paradigms, none of brain areas were found after 1 week of consolidation (Fig. 3C).Discussion
After learning, large-scale plasticity in the mouse brain were detected using resting-state fMRI. We observed that multiple functional areas are involved in both paradigms and intensive training (1-Day APA) induced more cortical networks, particularly related to sensorimotor function. This supports the multiple trace theory of memory consolidation. Moreover, the brain connectivity was reorganized after 1 week of consolidation in both 1-Day and 5-Day APA paradigms to be less hippocampal dependent, which is consistent with a theory of long-term memory consolidation. Different involvement of hippocampal formation in the connectivity at two time points suggests that the encoded information was redistributed to brain areas involved in long-term storage of memory. The reduced number of connections from post day 1 to post day 8 indicate ‘reorganization’ of brain connectivity during memory consolidation. Further studies are ongoing to determine how these brain areas are involved in memory consolidation. The findings would provide mechanistic understand of neuroplasticity and the role of resting-state networks in memory and dementia.Acknowledgements
No acknowledgement found.References
1. Nasrallah, F. A., To, X. V., Chen, D. Y., Routtenberg, A. & Chuang, K. H. Functional connectivity MRI tracks memory networks after maze learning in rodents. Neuroimage 127, 196–202 (2016).
2. Liu, J. et al. Intrinsic Brain Hub Connectivity Underlies Individual Differences in Spatial Working Memory. Cereb. Cortex 27, 5496–5508 (2016).
3. Muñoz-Moreno, E., Tudela, R., López-Gil, X. & Soria, G. Early brain connectivity alterations and cognitive impairment in a rat model of Alzheimer’s disease. Alzheimers. Res. Ther. 10, 16 (2018).
4. Kukushkin, N. V. & Carew, T. J. Memory Takes Time. Neuron 95, 259–279 (2017).
5. Dudai, Y., Karni, A. & Born, J. The Consolidation and Transformation of Memory. Neuron 88, 20–32 (2015).
6. Kandel, E. R., Dudai, Y. & Mayford, M. R. The molecular and systems biology of memory. Cell 157, 163–86 (2014).
7. Olton, D. S. & Paras, B. C. Spatial memory and hippocampal function. Neuropsychologia 17, 669–682 (1979).
8. Czajkowski, R. et al. Encoding and storage of spatial information in the retrosplenial cortex. Proc. Natl. Acad. Sci. U. S. A. 111, 8661–8666 (2014).
9. Walf, A. A. & Frye, C. A. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 31, 1097–1111 (2006).
10. Kim, E. J., Pellman, B. & Kim, J. J. Stress effects on the hippocampus: A critical review. Learn. Mem. 22, 411–416 (2015).
11. Lee, H. L., Li, Z., Coulson, E. J. & Chuang, K. H. Ultrafast fMRI of the rodent brain using simultaneous multi-slice EPI. Neuroimage 195, 48–58 (2019).
Figures