1954
Chemogenetic neuromodulation of striato-cortical direct pathway projections cause alterations in brain-wide network connectivity1Neural Control of Movement lab, DHEST, ETH Zurich, Zurich, Switzerland, 2Laboratory of Molecular and Behavioural Neuroscience, DHEST, ETH Zurich, Zurich, Switzerland, 3Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 4Institute of Pharmacology and Toxicology, University of Zurich, Zurich, Switzerland
Synopsis
RsfMRI is used to investigate healthy and diseased brains via temporal correlations of distinct brain regions i.e. functional connectivity (FC). However, this gives little insight into the contribution of specific cell-types to the recorded signals. To tackle this, we measured rsfMRI in mice while chemogenetically exciting or inhibiting D1 expressing cells in dorsomedial striatum (dmCP) via DREADDs. Initial results illustrate opposing effects of excitation and inhibition on FC, observed only within anatomically connected nodes of corticostriatal circuitry. These results are the first, necessary step for further disentangling the role of dmCP on motor control and behavior.
Motivation
Resting-state fMRI (rsfMRI) data demonstrate temporal correlations in the blood oxygen level-dependent signals (BOLD) of distinct brain regions, thus allowing us to study functional connectivity (FC) of the whole brain at rest 1. The past years have seen an exponential increase in rsfMRI studies investigating FC in health and disease, but with little insight into how different cell types affect BOLD signal and consequently FC. The striatum is the main input structure of the cortiostriatal circuitry; it consists of 95% GABAergic medium spiny neurons (MSN) that can be divided into two populations: (i) dopaminergic receptor type D1-expressing neurons and (ii) dopaminergic receptor type D2-expressing neurons 2-4. It is well known that these two pathways work in balance to accomplish a complex task of motion, while their disbalance has been related to various pathologies 5. Here, we study how a disbalance of D1 MSNs affects striatal FC. To tackle this, we chemogenetically manipulated the right dorsomedial striatum (dmCP) by either exciting or inhibiting D1-expressing MSNs and measured the changes via rsfMRI in mouse.Methodology
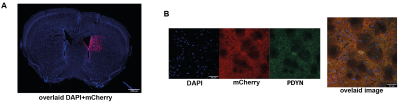
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) were used to target right dmCP (+0.5mm AP, +1.5mm ML, -3mm DV) in Drd1a-cre animals. Mice were injected with 950nl of either DIO-hM3Dq-mCherry (excitatory DREADD, n=14), DIO-hM4Di-mCherry (inhibitory DREADD, n=13) or DIO-mCherry (control virus, n=10). Four weeks later, to accommodate recovery and optimal viral expression, we performed an open field behavioural test. Mice were interperitoneally injected with 30 µg/kg of clozapine to activate the DREADD receptors; after 10 min they were placed in a squared open field arena and their behaviour was recorded for 25 min. Data was analysed using EthoVision software. A week later, rsfMRI measurements were acquired with a 7T Bruker Pharmascan scanner equipped with a dedicated mouse-brain receive-only cryogenic coil following well-established pipelines for animal handling, anaesthesia and data acquisition 6. An GE-EPI sequence was used with following acquisition parameters: repetition time TR=1 sec, echo time TE=15 ms, flip angle=60°, matrix size = 90x50, in-plane resolution = 0.2x0.2 mm2, number of slices = 25, slice thickness = 0.4 mm, 2280 volumes for a total scan of 38 min. Clozapine was intravenously injected 15 min after the scan start at the dose of 30 µg/kg. RsfMRI data was preprocessed using an already established pipeline for removal of artefacts from the time-series 7,8. At the end of the experiments, all mice were perfused and standard immunohistochemical analysis used to qualitatively validate the expression of the DREADD receptors and D1 MSN protein prodynorphin (Fig. 1A-B).Results & Discussion
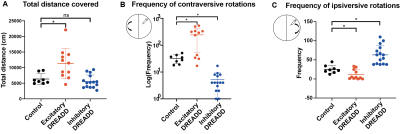
We first tested whether activating the DREADDs influenced behavior using the open field test. Exciting dmCP caused mice to increase motor activity and to increase contraversive rotations as compared to controls. By contrast, inhibition of dmCP did not affect overall motor activity but decreased contraversive rotations and increased ipsiversive ones, as compared to control mice. These results replicate previous findings9-11and confirm that our DREADD manipulation is effective (Fig. 2A-C).Changes in functional connectivity (FC) were measured between the dmCP and all other brain regions (n=131) using a sliding-window approach in non-overlapping intervals of one-minute. In each interval and for each pair of regions, we calculated the effect size (Cohen’s d) between excitatory and control groups (Figure 3A). During the first 15 minutes (baseline), Cohen’s d varied on average between –0.4 and +0.4 (null-to-small effect), and did not exhibit an appreciable spatial or temporal pattern. However, immediately after clozapine injection, we noticed a rapid change in behavior in a number of nodes connected to dmCP, showing a reduction in FC in the excitatory D1 mice as compared to controls (Fig. 3A). Decreased FC was observed in nodes including prefrontal cortex i.e. infralimbic (ILA), prelimbic (PL), orbitofrontal (ORB); anterior cingulate (ACA) and also retrosplenial (RSP) as well as somatomotor cortices (MO) (Fig. 3B). These nodes form anatomical projections to dmCP 12 and in control conditions exhibit a strong positive FC correlation with them. In these same nodes, we assessed the inhibition of D1-MSN. Interestingly, ACA,ORB and RSP show an increase in connectivity, although much weaker in the effect size (Fig 3C).
To the extent of our knowledge, this study is the first to describe that perturbing D1-MSN cells activity in the dmCP translates into whole-brain network connectivity changes. These results are the first, necessary step for further disentangling the role of dmCP on motor control and behaviour.
Acknowledgements
No acknowledgement found.References
1 Krishnan, G. P., Gonzalez, O. C. & Bazhenov, M. Origin of slow spontaneous resting-state neuronal fluctuations in brain networks. Proc Natl Acad Sci U S A115, 6858-6863, doi:10.1073/pnas.1715841115 (2018).
2 Bonnavion, P., Fernandez, E. P., Varin, C. & de Kerchove d'Exaerde, A. It takes two to tango: Dorsal direct and indirect pathways orchestration of motor learning and behavioral flexibility. Neurochem Int124, 200-214, doi:10.1016/j.neuint.2019.01.009 (2019).
3 Ferguson, S. M.et al.Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci14, 22-24, doi:10.1038/nn.2703 (2011).
4 Parent, A. & H., H. L. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews, 91-127 (1995).
5 Haber, S. N. Corticostriatal circuitry. Dialogues in Clinical Neuroscience 18(2016).
6 Zerbi, V.et al.Dysfunctional Autism Risk Genes Cause Circuit-Specific Connectivity Deficits With Distinct Developmental Trajectories. Cerebral Cortex, doi:10.1093/cercor/bhy046 (2018).
7 Zerbi, V., Grandjean, J., Rudin, M. & Wenderoth, N. Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage123, 11-21, doi:10.1016/j.neuroimage.2015.07.090 (2015).
8 Sethi, S. S., Zerbi, V., Wenderoth, N., Fornito, A. & Fulcher, B. D. Structural connectome topology relates to regional BOLD signal dynamics in the mouse brain. Chaos27, 047405, doi:10.1063/1.4979281 (2017).
9 Runegaard, A. H.et al.Modulating Dopamine Signaling and Behavior with Chemogenetics: Concepts, Progress, and Challenges. Pharmacol Rev71, 123-156, doi:10.1124/pr.117.013995 (2019).
10 Bay Konig, A., Ciriachi, C., Gether, U. & Rickhag, M. Chemogenetic Targeting of Dorsomedial Direct-pathway Striatal Projection Neurons Selectively Elicits Rotational Behavior in Mice. Neuroscience401, 106-116, doi:10.1016/j.neuroscience.2019.01.013 (2019).
11 Lee, H. J.et al.Activation of Direct and Indirect Pathway Medium Spiny Neurons Drives Distinct Brain-wide Responses. Neuron91, 412-424, doi:10.1016/j.neuron.2016.06.010 (2016).
12 Hintiryan, H.et al.The mouse cortico-striatal projectome. Nat Neurosci, doi:10.1038/nn.4332 (2016).
Figures