1950
Effect of anesthetics on spontaneous neural activity and functional connectivity in the bilateral somatosensory cortices in rats1Massachusetts General Hospital, Boston, MA, United States, 2Asan Medical Center, Seoul, Korea, Republic of, 3Portsmouth Abbey School, Portsmouth, RI, United States
Synopsis
Resting state fMRI study in animal model often involved use of anesthetics, but the effect of anesthetics on the spontaneous neural activity is not well known. We investigated how different types and doses of anesthetics (isoflurane and α-chloralose) influence the LFP activity and functional connectivity in comparison with the awake state. We observed distinct effects of these anesthetics on spontaneous neural activity (e.g. burst-suppression pattern and nonspecific correlation for high dose of isoflurane) and evoked response (e.g. potentified sensory-evoked response in bilateral cortices with α-chloralose). The effect of anesthetics should be taken into account in animal resting state fMRI studies.
Introduction
Resting state fMRI (rsfMRI) studies in animal models often involved use of anesthetics in order to prevent head movement.1,2,3,4,5 However, varying effects of different anesthetics on the spontaneous neural activity and associated alterations in the resting state functional connectivity which ultimately underlies rsfMRI findings are not well understood. For example, isoflurane often introduces less specific global functional connectivity in resting BOLD fMRI in animals, and it is unclear whether such global correlation arise from synchronized neural activity in the brain or just vasodilation effect of isoflurane. Here we aimed to investigate spontaneous brain activity and functional connectivity in the rat neocotex in various states of consciousness using in vivo electrophysiological recording. We compared resting state brain activity and functional connectivity in the varying level of consciousness using two commonly used anesthetics (isoflurane and α-chloralose) as well as in wakeful state.Methods
Adult male Sprague-Dawley rats (n = 8, 300-350 g) were used for the electrophysiology experiment. The animals were tracheotomized and mechanically ventilated at rate of ~40 cycles/min with monitoring of the heart rate and body temperature. The electrophysiological recordings were performed in the forelimb region of the bilateral primary somatosensory cortices (S1fl: 4.0 mm lateral and 0.5 mm anterior from the bregma) using two linear multi-electrode arrays (23 contacts along 2.2 mm for each electrode).6 The LFP was recorded with a sampling rate of 1,000 Hz under the following conditions: (1) for 5 min during rest and (2) for 2 min during forelimb stimulation (~1.0 mA, 3 Hz, 12 pulses per train, duration of each pulse of 0.3 msec, inter-train interval of 6 sec). We compared the LFP activity during (i) awake (pancuronium only), (ii) anesthetized states with varying dose of isoflurane (0.5%, 1.0%, 1.5% and 2.0%) and (iii) α-chloralose (30 mg and 60 mg/kg/hr).Results
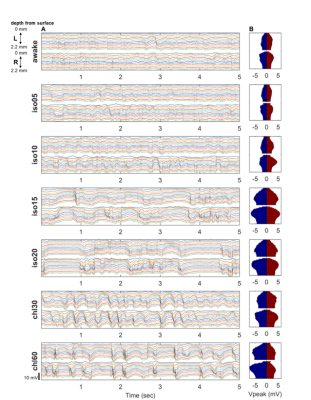
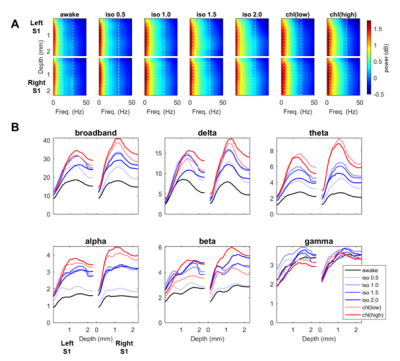
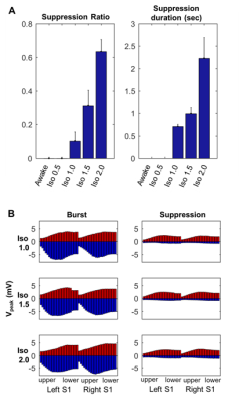
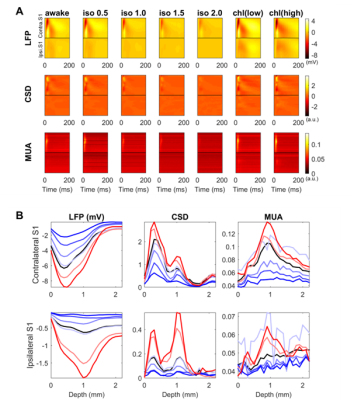
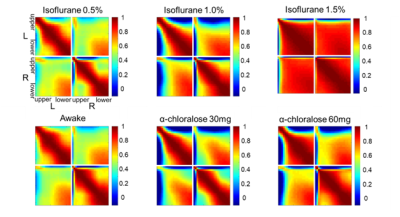
Spontaneous LFP activity during different anesthesia conditions are shown in Fig. 1. When compared to the awake activity, both types of anesthesia increased the overall amplitude of spontaneous LFP activity, particularly the slow waves in deep layers. In general, neuroelectric characteristics found in the low dose isofluane (0.5%) was highly similar to those seen in the awake state. In spectral power, delta, theta, alpha and beta band powers were modulated by anesthetic conditions but gamma band power did not change significantly (See Fig. 2). Slow wave activity (delta and theta bands) was smallest in awake state, and increased with isoflurane in dose-dependent manner. α-chloralose also increased slow wave activity, and theta band power was particularly higher compared to isoflurane anesthesia. High dose of isoflruane (≥ 1.5 %) induced the burst-suppression pattern, and the duration of suppression period increased with the isoflurane dose as shown in Fig. 3. On the other hand, animal anesthetized with α-chloralose did not exhibit such burst suppression pattern in spontaneous neural activity. Intersestingly, isoflurane and α-chloralose resulted in opposite effect in cortical excitability to sensory stimuli (see Fig. 4). High dose of isoflurane decreased sensory-evoked response, exhibiting rapid neural habituation for repeated stimuli. α-chloralose did not diminish the evoked response, but rather intensified the evoked response (particularly stronger for ipsilateral S1). We estimated functional connectivity within unilateral somatosensory cortex and between bilateralsomatosensory cortices using zero-lag correlation as shown in Fig. 5. Awake state showed the weakest interhemispheric correlation between bilateral cortices, particularly in upper layers (depth of 900 um or less). Isoflurane with dose of 1.0% or higher gradually introduced global correlation in bilateral cortices. Interhemispheric correlation was nonspecifically increased in all layers, and it might be associated with large slow wave and burst-suppression observed in isoflurane anesthesia. Layer-specific correlation pattern was largely disrupted in anesthesia under isoflurane, but moderate dose of α-chloralose (~30 mg/kg.hr) conserved layer-specific pattern in functional connectivity.Discussion
Anesthetics largely affected on spontaneous neural activity except only light sedation with low dose of isoflurane (0.5%). Isoflurane (1.0% or higher dose) resulted in strong, nonspecific increase of interhemispheric correlation while α-chloralose increased the overall correlation maintaining the laminar specificity which are also observed in low-dose isoflurane and awake state. Burst-suppression with high dose of isoflurane might be associated with the neural inhibition/disinhibition mechanisms and could induce habituation of the sensory-evoked responses.7,8 α-chloralose did not exhibit similar burst-suppression behavior and retained neural sensitivity to the external stimuli (e.g. forepaw stimulation). Both anesthetics increased the slow wave activity concentrated around layer 5, which could be associated with the sensory gating during anesthesia. Although differently characterized, both anesthetics affected the LFP activity and functional connectivity outcomes, for which thorough investigation of such effects are warranted prior to the application of rsfMRI for studying neural networks in anesthetized animals. For example, a resting fMRI study in rats with varying dose of isoflurane (1.0~1.8%) reported non-specific global correlation of BOLD fMRI signal,4 but light sedation with isoflurane (~0.5%) was reported to affect less on gross functional connectivity pattern in resting state fMRI signal in rats in accordance with our findings.9Acknowledgements
This research was supported by grants from the National Institutes of Health (1R21EY02637901 and 5R01MH11143802), Seoul R&D Program (FI170002), and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2017R1D1A1B03030713 and 2018R1A6A3A01013571). We thank the Biomedical Computing core facility at the ConveRgence mEDIcine research cenTer (CREDIT), Asan Medical Center for their technical support and instrumentation.References
1. Lu H et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. PNAS. 2007:104(46):18265-18269.
2. Liu X et al. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cerebral Cortex. 2011;21(2):374-384.
3. Pan, W.-J et al. Broadband local field potentials correlate with spontaneous fluctuations in functional magnetic resonance imaging signals in the rat somatosensory cortex under isoflurane anesthesia. Brain connectivity. 2011; 1(2):119-131.
4. Liu, X et al. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain topography,. 2013; 26(3):363-377.
5. Gorges M et al. Functional Connectivity Mapping in the Animal Model: Principles and Applications of Resting-State fMRI. Frontiers in Neurology. 2017; 8:200.
6. Baek K et al. Layer-specific interhemispheric functional connectivity in the somatosensory cortex of rats: resting state electrophysiology and fMRI studies. Brain Structure and Function. 2017; 221(5):2801-2815.
7. Saleem, A. B et al. Methods for predicting cortical UP and DOWN states from the phase of deep layer local field potentials. Journal of computational neuroscience. 2010; 29(1):49-62.
8. Sellers KK et al. Anesthesia differentially modulates spontaneous network dynamics by cortical area and layer. Journal of Neurophysiology. 2013;110(12): 2739-2751.
9. Stenroos, P., et al. Awake rat brain functional magnetic resonance imaging using standard radio frequency coils and a 3D printed restraint kit. Frontiers in neuroscience. 2018;12:54.
Figures