1947
Implementation and optimization of steady state free precession MRI for mapping BBB leakage in mouse brain at 9.4T1Radiology, University of Illinois at Chicago, CHICAGO, IL, United States, 2Anatomy and Cell Biology, University of Illinois at Chicago, CHICAGO, IL, United States, 3Research Resources Center, University of Illinois at Chicago, CHICAGO, IL, United States
Synopsis
In neurodegenerative diseases, e.g., Alzheimer’s disease, it is important to study cerebral blood flow (CBF) and blood brain barrier (BBB) permeability. Arterial Spin Labeling (ASL), used to measure CBF, is hampered by physiological variability, requiring many averages, and thus, fast imaging. Echo Planner Imaging, the most used fast imaging method, suffers from massive distortions and blurring for mice brain imaging at high field MRI. Alternatively, in this study we successfully implemented steady state free precession fast imaging for the measurement of the BBB leakage in mouse brain at 9.4T.
Purpose
Due to the increasing recognition of BBB as a pathogenic factor for neurological diseases, it is important to imaging Blood Brain Barrier (BBB) leakage in mouse models. Recently, Wang et al. had proposed a novel method for imaging BBB leakage using pseudo Continuous Arterial Spin Labeling (pCASL) to measure CBF and incorporating diffusion weighting (DW-ASL) to determine the relative permeability of water across the BBB[1]. This method was successfully demonstrated in human brain imaging at 3T based using an Echo Planner Imaging (EPI) readout. However, EPI for mouse imaging at 9.4T is associated with massive distortions and blurring, posing challenges for preclinical translation research. As an alternative fast imaging technique, Steady State Free Precession (SSFP) has been successfully implemented for ASL mouse imaging at high field [2]. In this study, we aimed to develop Magnetization Prepared Steady State Free Precession (MP-SSFP) MRI for imaging the mouse brain BBB leakage based on DW-ASL. During the sequence development, inversion time (TI), as a key parameter, was optimized to measure the relative amount of blood water transferred across the BBB before complete transfer has occurred.Methods
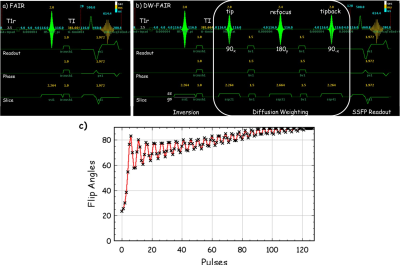
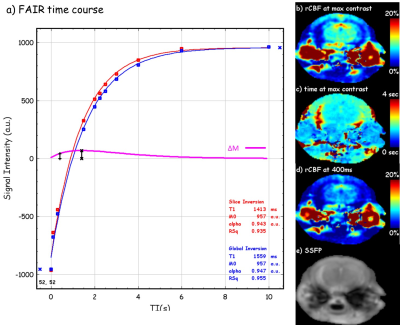
Three mice were scanned at a Varian horizon 9.4T small animal MRI scanner (Agilent Technologies, Santa Clara, CA) under an approved IACUC protocol for the IR and DW SSFP pulse sequence. The imaging parameters are, for CBF, TI = 0, 100, 300, 1500, 2000, 2250, 2500, 3000, 4000, 6000 ms, 20 repeats, slice thickness = 2x imaging thickness, or 2 mm; for BBB permeability, TI = 400ms, 150 repeats, b‑values = 0 and 50 s/mm2. The details for SSFP readouts are TR = 2 ms, TE = 1 ms, FOV = 18x18mm2, matrix = 128x128, slice thickness = 1 mm, and T1 recovery delay = 2.5 s.To measure the CBF and determine the earliest acceptable inversion recovery (IR) contrast difference ΔM between tagged and untagged blood water delivered to the imaging slice, a series inversion recovery (IR) SSFP [2] datasets with varied TI and alternating slice-selected inversion (SS) and global-selected (GS) inversion were collected. To capture the contrast from the MP and to obtain consistent actual flip angles, variable requested flip angles [3] was implemented for the SSFP readout at a centric k‑space ordering (figure 1d). Both SS and GS IR datasets with varied TI were fit to the T1 recovery equation $$$ S_ti = M_0(1-2α·exp(-ti/T_1) $$$ to estimate the apparent T1 (T1app), from global select data, and resolve the magnitude polarity near zero issue [4]. To measure the relative permeability of water across the BBB, an IR and DW combined MP-SSFP pulse sequence was developed (fig 1b). The relative BBB permeability was quantified from ΔMb50/ΔMb0, where ΔMb50 and ΔMb0 are the signal differences between SS and GS under diffusion b = 50 and 0 s/mm2, respectively.
Results
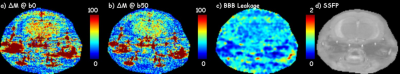
Figure 2 demonstrated the fitting of IR under SS and GS, the optimization of TI for acceptable signal difference at around 400 ms, and example CBF maps under the optimal TI. Figure 3 shows representative ΔMb0, ΔMb50, and the resultant BBB relative permeability map from a healthy mouse brain, and SSFP global select image for anatomical reference. The BBB leakage maps were found to be highly reproducible between the three mice scanned. Across the three mice, the relative permeability showed z‑scores (σ/μ) of 2.4%, 4.6%, and 3.1% in cortex, thalamus, and hippocampus, respectively, demonstrating high reliability for detecting small pathological changes of brain BBB in mouse at 9.4T.Discussion
This study has demonstrated the feasibility of using a magnetization prepared steady state free precession pulse sequence for measuring the relative BBB permeability in mouse at high field. After initial tests and optimization, the novel imaging sequence has been well prepared for future studies on detecting BBB leakage due to neurodegenerative diseases.Acknowledgements
This work was supported by NIH grants R21EB023516, R01AG061114, and R21AG053876.References
1) St. Lawarence, et.al., Magn. Reson. Med. 2012;67:1275-1284.
2) Gao, et.al, NMR Biomed, 2014;27:996-1004.
3) Scheffler, Concepts in Mag. Res. 1999;11:291-304.
4) Barral, et.al., Magn. Reson. Med. 2010;64:1057-1067.
Figures