1941
MRI assessment of vascular disruption in tau pathology animal model (rTg4510 mouse) of Alzheimer disease1School of Biomedical Convergence Engineering, Pusan National University, Busan, Republic of Korea, 2Department of Neurology, Massachusetts General Hospital and Harvard Medical School,, Boston, MA, United States, 3Department of Radiology, Asan Medical Center, Seoul, Korea, Republic of, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
We assessed vascular disruption in rTg4510 mouse (age of 6 and 9 months), a tau expressing transgenic animal model of Alzheimer’s disease, using MRI metrics such as vessel size index (VSI), Gd-DTPA leakge through BBB and water exchange index (WEI). The rTg4510 mice showed age-dependent expansion of the hippocampal lesion and profound vascular alteration around the hippocampal lesion: Increase in VSI, mild Gd-DTPA leakage and increased WEI. Our findings suggest the role of vascular components in development of Alzheimer’s disease with tau pathology. Vascular MRI assessments might have a potential application in early diagnosis and monitoring of Alzheimer’s disease.
Introduction
Vascular disruption including the breakdown of blood-brain barrier (BBB) has been implicated in pathology of Alzheimer’s diesease,1,2 but less attention was given so far. The rTg4510 trasngenic mouse is an animal model with tau pathology of Alzheimer’s disease which expressing human tau protein containing the P301L mutation. Previous MRI studies reported structural change such as severe atrophy of hippocampus and neocortex and reduced fractional anisotrophy values in the white matter.3,4,5,6 Functional and neurochemical alterations are also measured with manganese-enhanced MRI and magnetic resonance spectroscopy (MRS).6,7,8,9 There are few MRI studies on vascular disruption in the rTg4510 mouse model except only a couple of studies assessing vascular reactivity and cerebral blood flow (CBF) .10,11 In our preliminary study, we observed vascular remodeling in the rTg4510 mice (age of 9 months or older) with optical imaging of ex vivo brain tissues. Here we aimed to examine the change in vascular properties, e.g. BBB integrity, vessel size index (VSI) and CBF, in the rTg4510 mice at age of 6~ 9 months so that we can establish whether such BBB integrity and VSI metrics can be used as a MRI biomarker for early detection and longitudinal monitoring of Alzheimer’s disease developing with tau pathology.Methods
Nine female rTg4510 transgenic mice (N = 6 for 6-month-old, N = 3 for 9-month-old) and 9 nontransgenic control mice at the same age were used. MRI scan was conducted in 9.4T Bruker smallbore scanner with a Paravison console 5.1 in Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital (MGH). Animal was anesthetized with isoflurane 1.0~1.5% and the tail vein was cannulated for the contrast agent injection (Gd-DTPA and SPION). CBF was estimated using gradient-echo EPI scans with continuous aterial spin labeling (ASL) with following parameters: TR = 3700 ms, TE = 14.45 ms, number of echoes = 8, flip angle = 90 deg, matrix size = 64 x 64, FOV = 16 x 16 mm, number of slices = 16, slice thickness = 0.5 mm, labeling duration = 3000 ms, labeling slice offset = 15 mm. Multi-echo T2- and T2*-weighted images were acquired using MSME and MGE sequences (TR/TE = 2500/15 ms and 1500/3 ms, flip angle = 90 deg and 30 deg, respectively) with the same FOV as the EPI. BBB leakage (Gd-DTPA) and VSI were estimated using the MSME and MGE images acquired before and after contrast agent injection: 1st) Gd-DTPA (0.2 mmoles Gd/kg), 2nd) SPION (~30 mg/kg). Water exchange index (WEI)12 was estimated using M0 intensity estimated from 3D MGE sequence with the following parameters: TR = 30 ms, TE = 2.5 ms, number of echoes = 8, matrix size = 100 x 64 x 64, FOV = 25 x 16 x 16 mm, flip angle = 20, 30 and 80 deg.Results
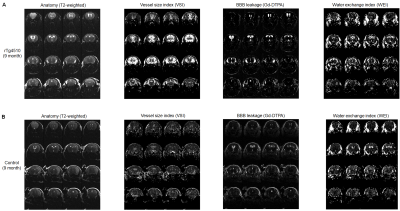
The rTg4510 mice exhibited small to moderate size of hippocampal lesion at the age of 6 months and much larger lesion at the age of 9 months. At the age of 9 months, cortical atrophy (thinning of the cerebral cortex) and enlaged ventricles are observed as well. As shown Fig. 1 and 2, vascular properties measured with MRI were altered around the hippocampal lesion. The 6-month-old rTg4510 mice showed increased VSI (voxel-wise CBV / MVV ratio) around the lesion. Local Gd-DTPA leakage was observed around the hippocampal lesion as well. Water exchange index contrast (apparent CBV at flip angle 20 deg - apparent CBV at flip angle 80 deg) was also focal increase around the lesion. At the age of 9 months with much larger hippocampal lesion, the rTg4510 mice showed profound alteration in VSI and BBB permeability as shown in Fig. 2. These vascular disruption tends to occur earlier in larger areas than age-dependent expansion of hippocampal damage. The non-transgenic control mice did not show profound alteration except mild BBB leakage around ventral hippocampus and increased WEI around the ventricle area at the age of 9 months.Discussion
The present study demonstrated the clear signs of BBB breakdown in the tau pathology animal model of Alzheimer disease (rTg4510 mouse), i.e. Gd-DTPA leakage around the hippocampal lesion. This BBB disruption was also observed with increased water exchange index, which can be used for ealry detection of BBB breakdown.12 In addition, the rTg4510 mice exhibited increased VSI, which implies that vascular remodeling and increased vessel diameters in the affected region. In a preliminary optical imaging study on ex vivo brain tissues, our collaborators observed alteration in vascular structures, particularly increased vessel size, which is in accordance with the VSI finding. Our findings suggest the role of vascular components in development of Alzheimer’s disease with tau protein pathology.1 MRI assessments of vascular disruption might have a potential application in early diagnosis and monitoring of Alzheimer’s disease.Acknowledgements
This research was supported by grants from the National Institutes of Health (1R21EY02637901 and 5R01MH11143802), Seoul R&D Program (FI170002), and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2017R1D1A1B03030713 and 2018R1A6A3A01013571). We thank the Biomedical Computing core facility at the ConveRgence mEDIcine research cenTer (CREDIT), Asan Medical Center for their technical support and instrumentation.References
1. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011; 12(12):723-738
2. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018; 14(3):133-150
3. Xie Z et al. Characterizing the regional structural difference of the brain between tau transgenic (rTg4510) and wild-type mice using MRI. Med Image Comput Assist Interv. 2010;13(Pt 1):308-315.
4. Yang D et al. Volumetric MRI and MRS provide sensitive measures of Alzheimer's disease neuropathology in inducible Tau transgenic mice (rTg4510). Neuroimage. 2011;54(4):2652-2658.
5. Sahara N et al. Age-related decline in white matter integrity in a mouse model of tauopathy: an in vivo diffusion tensor magnetic resonance imaging study. Neurobiol Aging. 2014;35(6):1364-1374.
6. Wells JA et al. In vivo imaging of tau pathology using multi-parametric quantitative MRI. Neuroimage. 2015;111:369-378.
7. Kim J et al. Progressive pathological changes in neurochemical profile of the hippocampus and early changes in the olfactory bulbs of tau transgenic mice (rTg4510). Neurochem Res. 2017;42(6):1649-1660.
8. Perez PD et al. In vivo functional brain mapping in a conditional mouse model of human tauopathy (tauP301L) reveals reduced neural activity in memory formation structures. Mol Neurodegener. 2013;8:9.
9. Fontaine SN et al. Identification of changes in neuronal function as a consequence of aging and tauopathic neurodegeneration using a novel and sensitive magnetic resonance imaging approach. Neurobiol Aging. 2017;56:78-86.
10. Fisher EM et al. Increased cerebral vascular reactivity in the tau expressing rTg4510 mouse: evidence against the role of tau pathology to impair vascular health in Alzheimer's disease. J Cereb Blood Flow Metab. 2015;35(3):359-362.
11. Holmes HE et al. Imaging the accumulation and suppression of tau pathology using multiparametric MRI. Neurobiol Aging. 2016;39:184-194
12. Kim YR et al. In vivo quantification of transvascular water exchange during the acute phase of permanent stroke. Magn Reson Med. 2008;60(4):813-821.
Figures
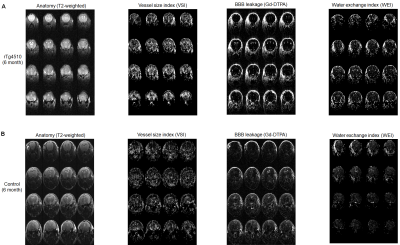
Figure 1. MRI measurement of Vascular properties in (A) rTg4510 mouse (tau pathology Alzheimer’s disease model) and (B) non-transgenic control mouse at the age of 6 months. rTg4510 mouse exhibited increased vessel size index, focal BBB breakdown and increased water exchange around the lesion in ventral hippocampus, but the control mouse did not.