1939
MR elastography and DTI for monitoring microstructural changes in the developing brain of Poly (I:C)-induced maternal immune activated rats.1Neuroscience Research Australia, Sydney, Australia, 2University of New South Wales, Sydney, Australia
Synopsis
Maternal infection during pregnancy can cause enlarged ventricles and compromise brain microstructure, which conventional imaging techniques cannot identify. We investigated the potential of magnetic resonance elastography (MRE) and diffusion tensor imaging (DTI) in detecting brain microstructural changes in offspring of Poly (I:C)-induced maternal immune activated rats. Results showed that DTI and MRE were sensitive to neurodevelopmental microstructural changes, but not to subtle longitudinal microstructural changes related to maternal infection. DTI showed that white matter injury differs between perinatal and adolescent Poly(I:C) rats, while MRE was not sensitive enough to detect subtle compromise of gray matter microstructure in Poly (I:C) rats.
Introduction
Maternal infection during pregnancy has been identified as a risk factor for neurodevelopmental psychiatric disorders in offspring, such as schizophrenia1. Some neurodevelopmental disorders cause enlarged lateral ventricles and smaller brain volumes that can be depicted accurately with conventional imaging techniques2, but not the early changes in brain microstructure that occur in both gray and white matter regions prior to the emergence of abnormal behaviour, and that may be treatable. Therefore, the aim of this study was to investigate the potential value of magnetic resonance elastography (MRE)3,4 and diffusion tensor imaging (DTI)5 in the detection of brain microstructural changes during growth in a rodent model of maternal immune activation. Previous studies have shown the capability of DTI and MRE to track brain microstructural changes in the white and gray matter during early postnatal development in healthy rats6,7.Methods
This study was approved by the local animal care ethics committee. We studied 12 male offspring of mothers in whom immune activation was stimulated during pregnancy by administration of the viral mimic polyriboinosinic-polyribocytidylic acid ((Poly (I:C)8, 4 mg/kg) on gestational day 15, plus 8 male control rats from saline-injected mother rats. All rats underwent MR imaging (9.4T, Bruker) at 4 and 10 weeks after birth, to characterise both early and late neurodevelopmental processes. T2-weighted MR images were collected to quantify the ventricle cross-sectional area. MR elastography (800 Hz) was used to measure cortical and caudate putamen shear modulus (G*). DTI (30 gradient directions, b=765.8 s/mm2, and 5 images, b =0 s/mm2) was conducted to quantify white matter (corpus callosum CC, internal and external capsules IC/EC) diffusion properties: fractional anisotropy (FA), mean diffusivity and axial/radial diffusivities (MD, AD and RD). Figure 1 shows typical images/maps obtained with this protocol. Immediately after the second scan, all brains were stained with luxol fast blue/cresyl violet to determine myelin and nuclei density in the white matter and NeuN/Iba-1 antibodies to quantify the number of neurons and microglia in the gray matter. Longitudinal data were analysed by fitting a mixed model or using a repeated measure two-way ANOVA followed by a Sidak's multiple comparisons test. At weeks 4 and 10, relationships between ventricle size, MR metrics and histological measurements (only for week 10) were assessed using Pearson correlations.Results
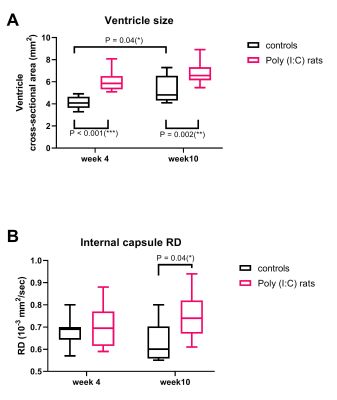
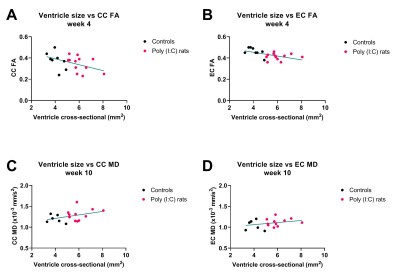
Ventricles were significantly enlarged in the Poly (I:C) rats from week 4 compared to controls (F(1,18)= 33.5, P<0.001), and were also larger at week 10 than at week 4 for controls (F(1,18)= 10.4, P = 0.005), but the interaction between group and week was not significant (F(1,18)= 0.5, P = 0.44, Figure 2A). IC RD was significantly higher in Poly (I:C) rats at week 10, but not at week 4 (F(1,33) = 4.5, P = 0.04, Figure 2B). All other MR metrics did not differ between groups at both time points, although cortical G*, CC FA/MD/AD, IC FA/AD, and EC FA/MD/RD/AD changed over time in both groups. At week 4, larger ventricles were associated with lower FA in CC and EC (r = -0.45, P = 0.04, and r = -0.53, P = 0.01, respectively, Figure 3 A and B). At 10 weeks, an increase in ventricle size was associated with increased white matter MR diffusion metrics (CC MD/AD, EC MD/RD/AD, Figure 3 C and D) and corpus callosum myelin content (r = 0.49, P = 0.04). An increase in cortical G* was associated with a decrease in local neuronal density (r = -0.64, P = 0.01). Ventricle size and histological variables were not associated with CP G* or IC diffusion measurements.Discussion
Ventricular enlargement was visible from week 4 in Poly (I:C) rats, but the expansion did not seem to progress, as the difference in ventricles size between groups was similar at week 10. This may be the reason why DTI and MRE could not detect microstructural changes over time in the Poly(I:C) rats, although they were sensitive to microstructural changes related to neurodevelopmental growth in both white and gray matter. However, enlarged ventricles were associated with decreased white matter FA in perinatal brains (week 4), and then with increased MD, AD and RD in adolescents/ young adults (week 10), suggesting that DTI may be useful to characterise white matter injury that may evolve during neurodevelopment in Poly (I:C) rats.Conclusion
DTI may be useful to improve our understanding of neuropathological processes occurring in white matter of schizophrenic brains by characterising temporal variations in white matter diffusion properties, as shown here in a rodent model that exhibits similar neurodevelopmental changes. Measured changes in diffusivity properties may reflect improper white matter development and deficiency in connectivity that underlie cognitive impairments. In contrast, MRE was not sensitive enough to detect subtle compromise of the gray matter microstructure in poly (I:C) rats.Acknowledgements
This study was funded by the Rebecca L. Cooper foundation. LB is support by the National Health and Medical Research Council of Australia. The authors would like to thank the Biological Resources Imaging Laboratory (Mark Wainwright Analytical Centre) and the Biomedical Imaging facility of the University of New South Wales (UNSW, Australia) for technical support.References
1. Khandaker GM, Zimbron J, Lewis G ,et al. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013; 43 (2): 239-257.
2. Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998; 172: 110-120.
3. Muthupillai R, Lomas DJ, Rossman PJ, et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. SCIENCE. 1995; 269 (5232): 1854-1857.
4. Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008; 21 (7): 755-764.
5. Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996; 201 (3): 637-648.
6. Pong AC, Jugé L., Bilston L.E., et al. Longitudinal measurements of postnatal rat brain mechanical properties in-vivo. J. Biomech. 2016; 49(9):1751-1756.
7. Cai Y, McMurray MS, Oguz I, et al. Use of High Resolution 3D Diffusion Tensor Imaging to Study Brain White Matter Development in Live Neonatal Rats. Front Psychiatry. 2011; 2: 54.
8. Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009; 33 (7): 1061-1079.
Figures