Laura-Adela Harsan1, Laetitia Degiorgis1, Julien Todeschi2, Lea Becker3, Maxence Thomas de la Pintière1, Victor Mathis3, Chrystelle Po1, and Ipek Yalcin3
1ICube: Engineering science, computer science and imaging laboratory, University of Strasbourg, Strasbourg, France, 2ICube: Engineering science, computer science and imaging laboratory, Neurosurgery Department, University Hospital Strasbourg, University of Strasbourg, Strasbourg, France, 3Institute de Neurosciences Cellulaires et Intégratives, University of Strasbourg-CNRS, Strasbourg, France
Synopsis
The main objective of this study is
to modulate and map the anterior cyngulate cortex (ACC) functional connectivity
(FC) pathways underlying depression development in a mouse model. We use the
optogenetic approaches to create the depression phenotype in mice, by
activating the pyramidal ACC neurons expressing Channel rhodopsin 2 (ChR). We
further use resting state functional MRI (rsfMRI) as non-invasive read-out of
the effects at the level of functional brain connectivity. Four consecutive
sessions of optogenetic ACC stimulation induced strong depression phenotype and
major modifications of the functional mouse brain connectivity including
perturbed mesocorticolimbic pathways and default mode network patterns
Introduction and objectives
Mood
disorders, including depression, are increasingly seen as brain circuit
pathologies rather than as region specific related disorders1.
Recent data from the literature have identified the anterior cingulate cortex
(ACC) as a key area of the affective
component of chronic
pain and a
major player for further development of depression2. However,
pathological perturbations of one brain area
are rarely confined to
a single locus;
instead, they often
spread to affect
other regions and
their connectional pathways.
The
main objective of this study is to modulate and map the ACC functional
connectivity (FC) pathways underlying depression development in a mouse model. We
use the optogenetic approaches to create the depression phenotype in mice3,
by activating the pyramidal ACC neurons expressing Channel rhodopsin 2 (ChR).
We further use resting state functional MRI (rsfMRI) as non-invasive read-out
of the effects at the level of functional brain connectivity. Finally, we perform
seed based analysis and graph theory approaches to identify the ACC functional
circuitry signatures underlying depression.Materials and methods
Genetically modified mice expressing
channelrhodopsin-2 and yellow fluorescent protein (Thy1-ChR2-YFP) in a subset
of pyramidal neurons were used for optogenetic - rsfMRI studies. The animals
(N=28) were subjected to: (i) surgery for glass fiber cannulas insertion along
the whole vertical span of the ACC (1.7
mm long, cannulas MRI compatible,
Doric Lenses) – allowing the light delivery via an optic fiber during
optogenetic stimulation - (Fig. 1). (ii) a baseline rsfMRI brain scan, 1 week
after cannula insertion; (iii) optogenetic stimulation for 30 minutes/day, during
4 consecutive days (repeated stimulations with 463 nm blue light) - inducing the
anxio-depressive phenotype; (iv) behavioral evaluation of the depressive
phenotype via standard “novelty suppressed feeding test”; followed immediately
by (v) a second rsfMRI session to evaluate the functional connectivity
features. 14 mice were subjected to “real” optogenetic stimulation and showed
strong depression phenotype, while 14 mice (controls) underwent the same
cannula implant procedures but the light was switched off during “dummy” stimulation
sessions. Mouse brain rsfMRI was performed with a
7T animal scanner
(Biospec 70/30, Bruker,
Germany) and a combination of a transmit – receive volume coil (86mm) and a mouse brain adapted loop
surface coil allowing the passage of the optogenetic cannulas (MRI, Bruker,
Germany). The rsfMRI data was acquired under medetomidine anesthesia – initial
bolus injection of 0.3 mg MD per kg body weight followed by continuous sc
infusion of MD at 0.3 mg per kg body weight per hour. A one-shot GE-EPI sequence (TE / TR = 15 ms / 2000
ms; resolution = 0.14 ×
0.21× 0.5 mm³; 500 volumes of 31 axial slices) was used for rsfMRI, This data
was spatially normalized on the Allen Mouse Brain Atlas and frequency filtered
(<0.01Hz). Results and Discussion
Four consecutive sessions of optogenetic ACC stimulation
induced strong depression phenotype and major modifications of the functional
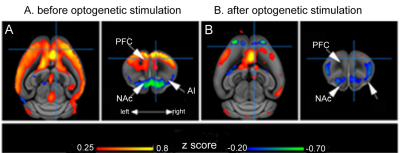
mouse brain connectivity. Fig 1 exemplifies the connectivity of dorsal ACC,
before (A) and after (B) optogenetic stimulations. Overall decrease of FC is
noticed between ACC and the rest of the cortical areas, notably prefrontal
areas (PFC) (red – positive correlations) but also with subcortical nuclei, such
as Nucleus Accumbens – NAc. Both PFC and NAc are known hubs of the
mesocorticolimbic circuitry, and their perturbed patterns
of connectivity in depression suggest
altered cognitive processing of motivation, aversion and reward. Additionally, our data
indicate a major impact of optogenetic stimulation on the default mode network
(DMN) pattern and on its cross-talk with the rest of the brain. Modifications of
the default-mode network
(DMN) is one
of the most widely replicated neuroimaging findings
in major depressive disorder (MDD) in humans4. Moreover, human fMRI
studies demonstrate that mood disorders
disrupt the relationship between DMN and the other
networks of the brain, similar to our observations in mice.Acknowledgements
No acknowledgement found.References
1Helm et al. Neuropsychiatr Dis
Treat. 2018 Oct 17;14:2715-2737; 2Rolls et al., Cereb Cortex. 2018
Nov 12. doi: 10.1093/cercor/bhy236; 3Barthas et al., Biol Psychiatry,
2015, 77:236-245.; 4Brakowski et al., J Psychiatr Res. 2017
Sep;92:147-159.