1931
Simple diffusion delivery via brain interstitial route for the treatment of cerebral ischemia1Department of Radiology, the First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Key Laboratory of Magnetic Resonance Imaging Equipment and Technique, Beijing, China, 3Department of Radiology, Peking University Third Hospital, Beijing, China, 4Institute of Medical Technology(IMT)of Peking University Health Science Center(PKUHSC), Beijing, China
Synopsis
Delivering pharmacologic agents directly into the brain has been proposed as a means of bypassing the blood brain barrier. Within this study we propose a novel system for delivering drugs into the brain named the simple diffusion (SDD) system. To validate this technique, rats were subjected to a sin- gle intracranial (at the caudate nucleus), or intraperitoneal injection, of the compound citicoline, followed two hours later by a permanent middle cerebral artery occlusion (pMCAO). These results suggest that given the appropriate injection point, through SDD a pharmacologically effective concentration of citicoline can be administered.
Introduction
A major obstacle in the development of drugs for use in the central nervous system is the ability to generate compounds capable of penetrating the blood brain barrier (BBB) [1]. One strategy, developed by Oldfield et al. [2], to overcome this problem is to bypass the BBB and infuse drugs directly into the CNS parenchyma through a process known as convection-enhanced delivery. With this technique a constant pressure gradient is maintained during interstitial infusion via one or several catheters and there is significantly enhanced distribution of a range of molecule sizes and an increase in the locoregional concentration of the infused compounds. Despite 16 years of basic research and multiple clinical trials using this technique being carried out on various CNS diseases [3], effective treatment has only been obtained in the brain tumor glioblastoma multiforme [4].Material and Methods
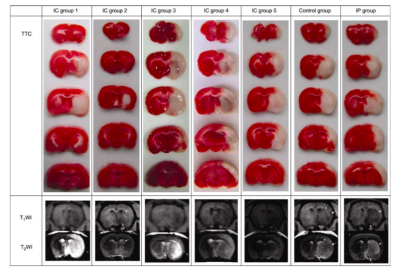
Citicoline (CDP-choline, cytidine 5′-diphosphocholine) was administered by intracranial microinjection into the caudate nu- cleus using the following stereotactic coordinates: bregma +1 mm, lateral 3 mm, vertical 4.5 mm (Lab Standard Stereo- taxic-Single, Stoelting Co., Illinois, USA). The study of the real-time detection of the infarction process by MRI and determination of infarct volume by TTC staining was com- posed of seven groups. For each group the animals (n= 8/group) received an intracranial (IC) microinjection of (i) 5 μL saline (control group), (ii) 0.00125 g kg−1 citicoline (IC group 1), (iii) 0.0025 g kg−1 citicoline (IC group 2), (iv) 0.00375 g kg−1 citicoline (IC group 3), (v) 0.005 g kg−1 citi- coline (IC group 4), (vi) 0.0125 g kg−1 citicoline (IC group 5). Animals in the final group received an intraperitoneal (IP) injection of 2 g kg−1 citicoline (IP group). Two hours after receiving an injection of citicoline (or saline), the animals were then subjected to a permanent middle cerebral artery occlusion (pMCAO). The choice to administer citicoline two hours prior to pMCAO was based on our previ- ous study on the diffusion pattern of Gd-DTPA in the brain extracellular space. We first made a model of permanent middle cerebral artery embolization in rats. Neuroimaging was performed using a 3.0T clinical MR system (Magnetom Trio, Siemens Medical Solutions, Er- langen, Germany). A wrist coil was used for axial T1W, T2W and DW images. And then Neurological deficit was evaluated.In addition, we performed TTC staining and infarct volume determination.Results
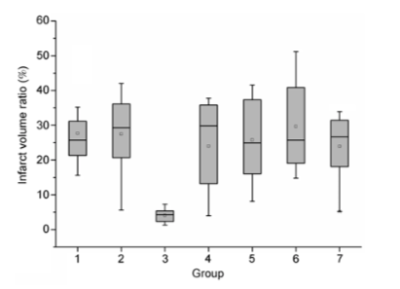
The results showed that IC administration of citicoline prior to pMCAO had a neuroprotective effect. These effects were confirmed at both the pathological (via TTC staining) and functional levels (via a Zea-Longa five-point scale). The best effect was observed in IC group 2 relative to all other groups (P<0.01, Table 1 and Figures 1 and 2). There was no statistical difference between IC groups 1, 3, 4 and 5, the IP group and the control group (P>0.05, Table 1 and Figures 1 and 2). On the other hand, there was a statistical difference between IC group 2 and the IP group (P<0.01, Table 1 and Figures 1 and 2). As observed in a previous study, behavioral deficits were correlated to the severity of the damage in the cortex. Therefore, the improved neural function might be attributed to a rescue of the penumbra in external cortex by citicoline administration, which was confirmed by the MRI findings (Figure 2). The TTC staining and MRI showed different localizations of ischemic lesions in six of the groups. The typical changes of decreased signal intensity on T1WI and increased signal intensity on T2WI, were well displayed in the infarct areas. Each group consisted of n=8 animals.Discussion and conclusion
In the present work, we have developed a simple diffusion delivery (SDD) technique, which has the potential to distribute the pharmacological agents more efficiently and less invasively within the brain parenchyma. Using this technique, a significant neuroprotective effect against cerebral ischemia can be achieved with a single injection of only a very small dose of the agent. The efficiency of SDD is based on the original injection point and the neuroprotective agents used. The design of the SDD system was based on brain inter- stitial MRI tracer imaging using the gadolinium derivative Gd-DTPA whereby MRI can be used to trace the diffusion and distribution of Gd-DTPA within the brain interstitial space. Our previous study found that using the caudate nucleus as the original injection point a single administration Gd-DTPA was able to diffuse to the MCA territory and where it remained almost unchanged for 2–4 h after the in- jection. In the current work, we assumed that a neuroprotective agent similar to Gd-DTPA would diffuse in the same way as Gd-DTPA. Among the many candidates, citicoline was chosen because it is similar to Gd-DTPA in respect to both molecular weight (510.31 Da vs. 938.02 Da) and polarity (both are hydrophilic). Additionally, because citicoline is an intermediate in the generation of phosphatidylcholine from choline in the normal brain, it is likely to be safer and more appropriate than other exogenous com- pounds for this local intracranial drug delivery studyAcknowledgements
No acknowledgement found.References
[1]Fisher M, Feuerstein G, Howells D W, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke, 2009, 40: 2244–2250.
[2]Bobo R H, Laske D W, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA, 1994, 91: 2076–2080.
[3]Debinski W, Tatter S B. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother, 2009, 9: 1519–1527.
[4]Gallia G L, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Cancer Netw, 2005, 3: 721–728
Figures