1930
Longitudinal Study of Resting State Connectivity in a Rat Model of Alcohol Use Disorder at Ultrahigh Fields1Department of Radiology, CMRR, University of Minnesota Medical School, Minneapolis, MN, United States, 2Departments of Neuroscience and Psychology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Resting state fMRI (rs-fMRI) has been used to investigate alcohol use disorder (AUD), mostly in humans; and a wide array of commonly induced neurological changes due to acute and chronic use have been reported. In this work, we established a rat model of AUD to longitudinally study the effects of chronic alcohol abuse on the functional brain connectivity using rs-fMRI at ultrahigh fields. Preliminary results revealed potential changes in reward-processing circuit connectivity and global brain activity. Further research is advised to quantify early effects observed in and between different neural structures (NAc/IBST/PreL/IL and ACC) during withdrawal stress and relapse.
Introduction
Alcohol use disorder(AUD) is an ongoing mental health problem with high recidivism due to wide availability and other factors. However, we still lack of knowledge about the plasticity in neural circuits that underlies persistent alcohol-induced behavior changes. Non-invasive fMRI approaches have the promise to detect changes in functional resting-state activity and neural connectivity(i.e. rs-fMRI), specifically in reward circuitry during different stages of AUD. Several neuroimaging studies have investigated the acute condition in humans and animal models at moderate blood alcohol levels(BAC).1,2 Furthermore, changes have been reported in humans in the default-mode network due to chronic alcohol abuse.3,4 Few alcohol rs-fMRI studies have been done in rodents longitudinally comparing acute conditions directly with longer chronic use, with some specific neurological deficits5, especially at high BAC.6,7 In this study, we established a longitudinal rat AUD model to study the reward circuitry during the acute intake, chronic consumption and withdrawal with relapse using rs-fMRI at ultra-high field strength.8Materials and Methods
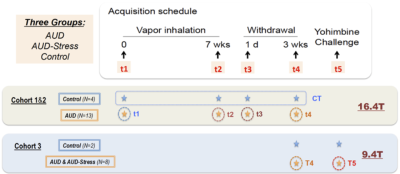
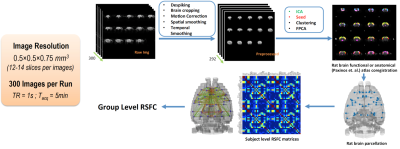
Rat AUD model and study design: Three cohorts of Sprague-Dawley rats (n=27, female) were used and each animal was scanned multiple times at different phases of AUD (see Fig. 1). After baseline acquisition (t1), rats underwent 7 weeks of daily 12h ethanol treatments in a vapor inhalation chamber (La Jolla Alcohol Research, California). Acute effects of alcohol intake were assessed right after the daily inhalation cycle at the end of 7-week exposure (t2) in cohorts 1&2. Acute alcohol-withdrawal effect was studied 6 hours after the daily alcohol exposure on the first day of withdrawal period (t3). After 3 weeks of abstinence from ethanol, animals were scanned again (t4), and in the same scan session, stress inducing chemical (yohimbine) or saline was given (i.p.), which is followed by another scan (t5) 30min later. The same scans were conducted before/after the yohimbine injection in cohort 3 rats, and additional behavioral tests (two-bottle choice) were performed prior the MRI (Fig. 2b). Blood alcohol contents were determined weekly with a standard alcohol dehydrogenase assay (Fig. 2a). All procedures were approved by IACUC of the University of Minnesota.Acquisition and analysis of rs-fMRI data: Spontaneously breathing animals were anesthetized (1.1-1.4% Isoflurane) and ear/bite bars were used to prevent head motion during the scan; animal core temperature was maintained at ~37±1°C via circulating water. Rats of cohort 1&2 and cohort 3 were scanned at 16.4T/26cm and 9.4T/31cm animal scanners (Varian Inc.), respectively. Custom-built RF surface coils (~3cm diameter) were used to acquire functional GE-EPI images (FOV 32x32mm2, in-plane resolution 0.5x0.5mm2, 12-14 1mm-slices, TE = 18 ms) with TR=1s and 300 volumes per series, and corresponding anatomic information. Pre-processing pipeline (Figure 3) includes brain extraction, motion correction, temporal bandpass filtering (0.01-0.2Hz) and gaussian smoothing (FWHM 1.5mm). The functional and anatomical MRI data were co-registered to a standard rat brain atlas. Seed-based analysis was performed using custom-built Matlab analysis programs and brain connectivity matrices were generated and evaluated.
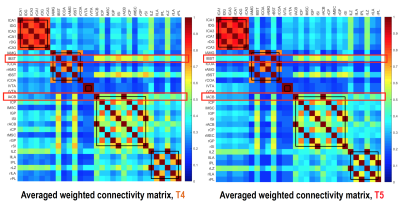
Results
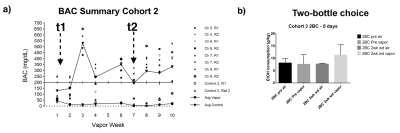
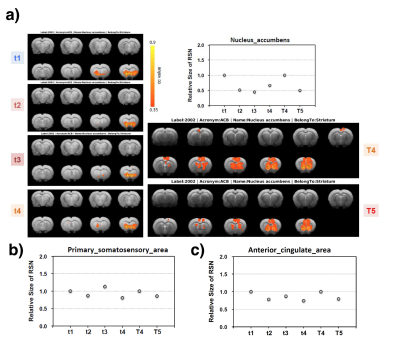
Figure 2a shows an example BAC information obtained from Cohort2 rats. Acute blood alcohol levels at t2 were within the target range of ~300 mg/dL, while animals displayed reduced ability to maintain their body temperature. At short-term abstinence (t3), ethanol (32 mg/dL) was almost cleared. The two–bottle test indicated that the rats in Cohort3 had an increased uptake of alcohol by preference (Figure 2b). Preliminary analysis of the rs-fMRI data lead to following observations: after normalizing to the baseline condition (t1), an acute exposure to alcohol (t2) resulted in a globally reduced resting-state network (RSN) activity or RSN sizes. Similar to t2, but an apparent return towards the baseline during short-term withdrawal (t3) was observed. Some hyperactivity in arousal networks (e.g. somatosensory Figure 4b) was also appeared during t3. After a longer withdrawal period, we saw a general reduction in activity (t4) and the connectivity between NAc-PreL/IL as well as in ACC was changed especially in T4 and T5 (Figure 4a). Furthermore, the third Cohort rats showed a stress response that may alter the rsfMRI connectivity (T4-T5) between the Nucleus Accumbens and BSTN (see Figure 5).Discussion and Conclusions
We designed a comprehensive longitudinal protocol to study changes in RSN at different stages of AUD using a rodent model to understand the alcohol-induced neural plasticity. Exposed animals produced changes in resting-state network on a global and reward-specific network level (e.g., BSTN-NAc seen in cohort 3 rats). It was expected in the acute condition (t2) that an additive effect of anesthesia and acute alcohol may occur, but it was rather surprising that some RSN under the acute withdrawal (t3) condition was not only recovered but also went beyond baseline levels. Functional connectivity changes lasted far longer than the periods of alcohol exposure and preliminary analysis showed stress response during late withdrawal stages (T5-T4). One note is that the usage of female rats in this study in some animals created challenges in maintaining stable physiological conditions and we observed more variations due to the longitudinal study across all scan sessions. With the sample size and variations observed in some individual single-animal brain responses between sessions this study provides valuable insights and motivate more research. Future investigations could benefit from whole head imaging coverage and more stable preclinical model of AUD.Acknowledgements
NIH P41 EB027061, P30 NS076408, S10 RR025031, the WM KECK Foundation and funding by Mitsubishi Tanabe Pharma Corp.References
1.
Esposito
F, Pignataro G, Di Renzo G, et al. Alcohol increases spontaneous BOLD signal
fluctuations in the visual network. NeuroImage. 2010;53(2):534-543.
2.
Khalili‐Mahani N, Zoethout RMW, Beckmann CF,
et al. Effects of morphine and alcohol on functional brain connectivity during
“resting state”: A placebo-controlled crossover study in healthy young men. Hum
Brain Mapp. 2012;33(5):1003-1018.
3.
Lei
X, Wang Y, Yuan H, Mantini D. Neuronal oscillations and functional interactions
between resting state networks. Hum Brain Mapp. 2014;35(7):3517-3528.
4.
Correas
A, Cuesta P, López-Caneda E, et al. Functional and structural brain
connectivity of young binge drinkers: a follow-up study. Sci Rep. 2016;6:31293.
5.
Chen
X, Zeng J, Shen Z-W, Kong L, Zheng W. Diffusion Kurtosis Imaging Detects
Microstructural Changes in the Brain after Acute Alcohol Intoxication in Rats.
BioMed Res Int. 2017.
6.
Adalsteinsson
E, Sullivan EV, Mayer D, Pfefferbaum A. In Vivo Quantification of Ethanol
Kinetics in Rat Brain. Neuropsychopharmacology. 2006;31(12):2683-2691.
7.
Logrip
ML. Molecular tools to elucidate factors regulating alcohol use. Alcohol. 2019
Feb;74:3-9.
8. Hearing MC, Jedynak J, Ebner SR, et
al. Reversal of morphine-induced cell-type–specific synaptic plasticity in the
nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci.
2016;113(3):757-762.
Figures