1929
Diffusion tensor imaging reveals persistent microstructural alterations up to six month in an open-head traumatic brain injury1Queensland Brain institute, The University of Queensland, Brisbane, Australia
Synopsis
Traumatic brain injury (TBI) is a severe problem worldwide. The non-invasive investigation of the microstructural alterations is of significant benefit for early diagnosis and interventions. In this study, we applied diffusion tensor imaging (DTI) to monitor the longitudinal microstructural changes in a controlled cortical impact rodent model of TBI from 2 hours and up to 6 months post-injury. Using DTI, we observe ongoing white matter changes following TBI, that initiate in corpus callosum and injury location at early timepoints and persists to exist up to 6 months, suggesting the temporal sensitivity of DTI to detect ongoing microstructural changes following TBI.
Background
Traumatic brain injury (TBI) is an external force applied to the brain causing a shearing force between white and grey matter initiating microstructural changes and neuroinflammatory responses1–4. Several studies aimed to develop non-invasive techniques to monitor microstructural changes following TBI for better diagnosis and possible treatment interventions. Diffusion tensor imaging (DTI) was successfully used to investigate the microstructural changes following the controlled cortical impact (CCI) rodent model of TBI5, showing reduced fractional anisotropy (FA) at widespread ipsilateral regions that persist to 4 weeks, in association with demyelination and microglial activity6. To date, no previous studies investigated the temporal profile of TBI in the CCI model using MRI up to 6 months. The primary objective of this study is to detect the spatiotemporal profile of microstructural alterations following CCI-TBI.Methods
A total of 37 male Sprague-Dawley rats underwent sham or severe-TBI using CCI surgery (speed: 5m/sec, depth: 2mm, time:200msec). The animals were randomly divided as a subset of n=10 TBI and n=10 sham scanned at 2 hours, 1, 3, 7, 14, 30, and 60 days post-TBI; and subset of n=9 TBI and n=8 sham scanned at 6 months post-TBI. Animals were scanned using a 9.4T MRI system (Bruker, Germany), with T2 weighted (rapid-relaxation-with-enhancement, TR/TE=5900/65ms, RARE-factor=8, FOV=32×25x20 mm, and matrix=256×256×40), and DTI to investigate the microstructural changes following TBI (axial, TR/TE/FA=10000ms/29ms/90o, FOV=24.8×24.8 mm, matrix=108×108×41, slice thickness/gap=0.5/0.1mm, 32-directions with b-values=750 and 1500s/mm2, and 4 b0-volumes). DTI data were corrected for eddy current, skull stripped7, and field bias; and DTI parameters were generated using FSL-DTIFIT. T2 and DTI images were normalized to the Schwarz template8 non-linearly using ANTS9. At each timepoint, differences between TBI and sham animals were calculated using unpaired t-test performed with permutation test (1000 permutations; corrected using family-wise error (a<0.05)).Results
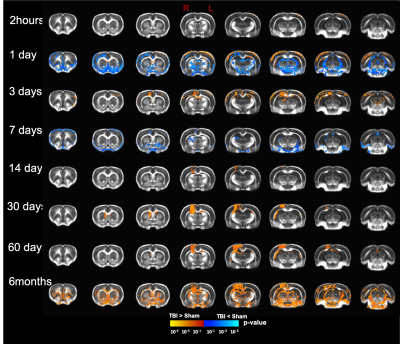
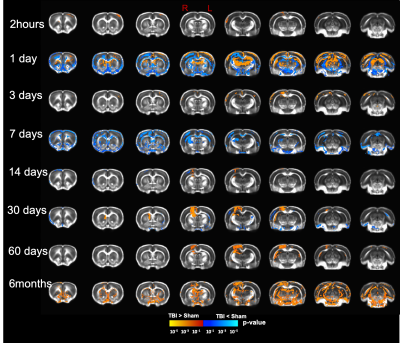
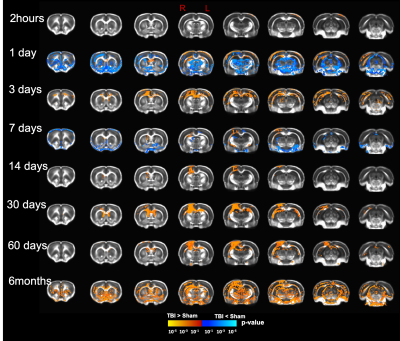
Our T2 images showed increased lesion volume with time with increased amounts of edema accumulation at the injury location (Fig 1). DTI showed alterations in the white-matter tracts at all timepoints initiating in the ipsilateral hemisphere and propagating to affect the contralateral hemisphere. Our results revealed reduced fractional anisotropy (FA) in the corpus callosum (CC) at 2hours; in CC, cingulum, and external capsule (EC) at 1 and 3 days; in CC, EC, internal capsule (IC), anterior commissure (AC), and cingulum at 7 and 14 days; with more FA reduction at 30 days in most of the white-matter tracts including CC, IC, EC, AC, and optic-tracts; reduced FA at the CC and optic tracts at 60 days; and in CC, AC, EC, and IC at 6 months (Fig 2). Mean diffusivity (MD) was reduced in AC, EC, and IC at 1 and 7 days; and increased MD in CC, IC, EC and OP at 6 months (Fig 3). Axial diffusivity (AD) was reduced in CC, AC, EC, and IC at 1 and 7 days; and in EC at 30 days; increased AD in CC, IC, EC and OP at 6 months; with no significant differences at the white-matter tracts at 2 hours, 3, 14, and 60 days (Fig 4). Radial diffusivity (DR) was persistently increased in CC from 1 day to 6 months; and in EC, AC, and IC at 6 months (Fig 5). The lesion area showed reduced FA from 1 day to 6months; while the MD and RD showed no difference at 2 hours, reduced at 1 day, and increased from 3 days to 6months; and the AD showed no difference at 2 hours, reduced at 1, 3, and 7 days, and increased from 14 days to 6months. Furthermore, FA was increased in the cortex, thalamus, and hippocampus at 1 day, followed by reduced FA values from 7 days to 6 months in cortex, thalamus, and hippocampus. The FA changes were associated with increased AD in the hippocampus, thalamus, hypothalamus, and brainstem at 1 and 60 days and 6 months; and reduced AD in cortex at 1,7 and 30 days. Furthermore, MD and RD were reduced in cortex, hippocampus, amygdala, and thalamus at 1 and 7 days; and MD and RD were increased in cortex at 3 days which extended to hippocampus, amygdala, hypothalamus, basal ganglia, and thalamus at 6 months.Discussion
To the best of our knowledge, this is the first longitudinal study to show ongoing changes in DTI up to six months in the wild type rat brain following a CCI injury. In the acute phase, increased FA in the grey-matter and reduced FA in the white-matter was a prominent feature; however, at the subacute and chronic phases (7 days up to 6months )of the injury progression, a reduction in FA in both grey- and white-matter was observed. The changes we see may be representative of increased primary microglial activity at the early timepoints associated with apoptosis and the start of demyelination followed by secondary microglial peak at the chronic phase associated with more demyelination4,6. These findings inform on the temporal dynamics of an injury in the rat brain revealed by DTI suggesting that persistent changes in the brain may be associated with long-term consequences. Further study to identify the associated underlying pathology at six months is under investigation which will promote better interpretation of human DTI findings following injury.Acknowledgements
This work as supported by Motor Accident Insurance Commission (MAIC) (Grant:2014000857), the Queensland Government, Australia for the research grant to FN. We thank the Australian Government support through NCRIS and the National Imaging Facility for the operation of 9.4T MRI at Centre of Advanced Imaging, University of Queensland, Brisbane, Australia.References
1. Tang-Schomer MD, Patel AR, Baas PW, Smith DH. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 2010;24:1401–1410.
2. Blennow K, Brody DL, Kochanek PM, et al. Traumatic brain injuries. Nat Rev Dis Prim. 2016;2:16084.
3. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol [online serial]. Elsevier Inc.; 2013;246:35–43. Accessed at: http://dx.doi.org/10.1016/j.expneurol.2012.01.013.
4. Loane DJ, Byrnes KR. Role of Microglia in Neurotrauma. Neurotherapeutics. 2010;7:366–377.
5. Osier ND, Dixon CE. The controlled cortical impact model: Applications, considerations for researchers, and future directions. Front. Neurol. 2016. p. 7:134.
6. Harris NG, Verley DR, Gutman BA, Sutton RL. Bi-directional changes in fractional anisotropy after experiment TBI: Disorganization and reorganization? Neuroimage [online serial]. Elsevier Inc.; 2016;133:129–143. Accessed at: http://dx.doi.org/10.1016/j.neuroimage.2016.03.012.
7. Chou N, Wu J, Bai Bingren J, Qiu A, Chuang K-H. Robust automatic rodent brain extraction using 3-D pulse-coupled neural networks (PCNN). IEEE Trans Image Process. 2011;20:2554–2564.
8. Schwarz AJ, Danckaert A, Reese T, et al. A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: Application to pharmacological MRI. Neuroimage. 2006;32:538–550.
9. Tustison NJ, Avants BB. Explicit B-spline regularization in diffeomorphic image registration. Front Neuroinform. 2013;7:39.
Figures