1926
Functional Reorganization and Behavioral Recovery in Rats with Repetitive Closed-head Injury after Drug Treatment1Neuroscience Research Center, Taipei Medical University, Taipei, Taiwan, 2Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 3Translational Imaging Research Center, Taipei Medical University Hospital, Taipei, Taiwan, 4Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 5Department of Biomedical Imaging and Radiological Science, China Medical University, Taichung, Taiwan, 6Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan
Synopsis
Significant improvement of locomotor activity and anxiety-like behavior along with reorganization of motor and default mode network was observed after NAC or NAC plus MINO treatment after repetitive closed-head injury, suggesting tentative treatment using drugs in patients with repetitive mild traumatic brain injury.
Introduction
We have implemented a repetitive closed-head injury (CHI) rat model1 to replicate repetitive mild traumatic brain injury (rmTBI), which has been recognized lately by the large patient population, enormous cost, and wide symptom spectrum.2 To date, there is no specific drug to manage TBI since moderate to severe TBI produces injury in both gray and white matter; in contrast, mTBI may show symptom only with less structural damage.3, 4 The previous studies have shown the treatment effect of N-acetylcysteine (NAC), the potent antioxidant undergoing first-pass metabolism to cysteine and cystine, or minocycline (MINO), a tetralcycline antibiotic agent, as individual drugs in animals with TBI.5, 6 Lately, the combination of NAC and MINO further demonstrated improved learning and memory behavior and histological outcome after TBI.7, 8 Herein, rather than using the animal models with focal brain contusion, we examine whether these two agents are effective to treat rmTBI and further explore the change of functional connectivity after drug treatment. With our closed-head injury (CHI) model,1 we focused on the treatment effect on the motor and anxiety-like behavior in the chronic phase after rmTBI and demonstrated the change of brain connectivity in the motor and default mode network (DMN), correspondingly.Methods
Male Sprague–Dawley rats were anesthetized with Chloral Hydrate for sham surgery (Sham group, n=13) or two CHI injury within 1 h targeting on the skull on top of the left sensorymotor cortex (n=15).1 Three intraperitoneal injections of saline (CHI group, n=5), NAC (75 mg/kg; NAC group, n=5), or MINO (22.5 mg/kg) plus NAC (75 mg/kg; NAC+MINO, group n=5) at 1 h, one and two days after the last injury.8 The open field test and longitudinal MRI was performed pre and at day 50 after CHI. Animals were anesthetized under the cocktail protocol with dexmedetomidine and isoflurane for MRI on a Bruker 7 T PharmaScan.9, 10 rsfMRI was acquired using the single-shot echo-planar imaging (EPI) with TR/TE= 1000/15 ms, FOV= 3.5 ×3.5 cm, matrix size = 64×64, 16 slices, slice thickness of 1 mm and number of repetition= 300 for total scan time= 5 min. T2-weighted images with the same geometry were also acquired. rsfMRI data analysis followed the previous published protocol.9, 10 Two-sample t-test and one-way Anova was used to determine the differences between pre- and post-rmTBI, and different drugs (p<0.05), respectively.Results & Discussion
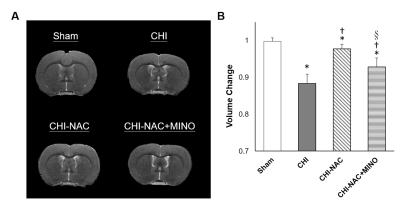
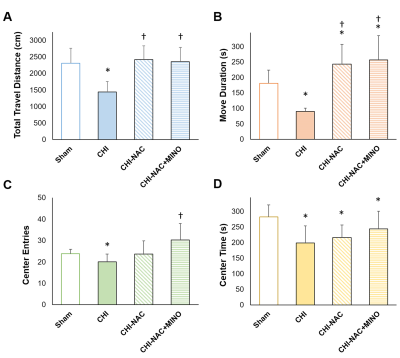
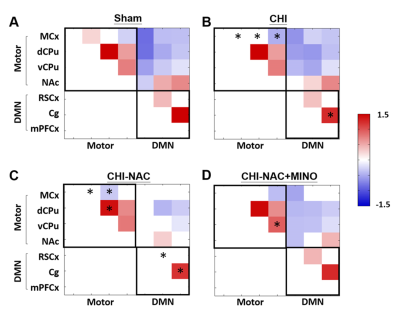
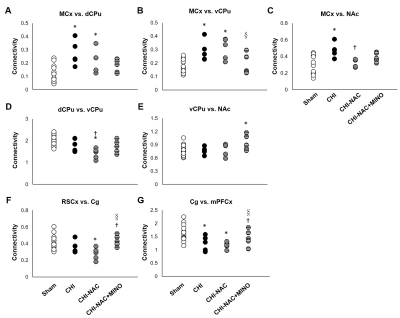
At 50 days after repetitive CHI, while no brain lesion including hemorrhage or tissue damage was observed (Fig. 1A), significant cortical atrophy was detected compared with baseline (Fig. 1B). Significant less volume loss was found after drug treatment. Although significant shorter total travel distance and shorter move duration were observed after repetitive injury, treatment using NAC and NAC plus MINO effectively recovered the motor deficit (Fig. 2A & B). Treatment with NAC plus MINO also successively restored the anxiety-like behavior indicated by the number of center entries (Fig. 2C), but not the center time (Fig. 2D). Our results showing behavior improvement after treatment may correlate to the previous study showing prevented myelin loss after treatment in the corpus callosum and hippocampal commissure.7 In addition, we observed different degrees of functional reorganization in motor network and default mode network in animals with and without drug treatment after repetitive CHI compared with the sham group (Fig. 3). Drug treatment with NAC plus MINO restored the hyper-connectivity between the motor cortex and other motor nuclei in the subcortical region (Fig. 4A-C). While no significant different was observed after CHI compared with sham group among the putamen and the nuclear accumbens, treatment with NAC weakened the connectivity between the dorsal and ventral CPu (Fig. 4D); treatment with NAC plus MINO increase the connectivity between ventral CPu and NAc (Fig. 4E). In the DMN, although hypo-connectivity was observed after treatment with NAC (Fig. 4F), successful restoration of connectivity was shown after treatment with NAC plus MINO (Fig. 4G). Our results in line with the clinical findings suggesting the decreased DMN functional connectivity associated with increase posttraumatic stress disorder11 may indicate that the restoration of DMN connectivity may serve as the index showing the amelioration anxiety or stress. Our future work will investigate how functional connectivity change in these networks correlates with behavioral outcome after repetitive CHI and whether the correlation will be altered by drug treatment.Acknowledgements
This study was funded in part by Ministry of Science and Technology (MOST 108-2314-B-038-002 and MOST 106-2218-E-039-001-MY3), Taipei, Taiwan.References
1. Kao Y J, Lui Y W, Lu C F, et al., Behavioral and Structural Effects of Single and Repeat Closed-Head Injury. AJNR Am J Neuroradiol, 2019.
2. Aungst S L, Kabadi S V, Thompson S M, et al., Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab, 2014. 34(7): 1223-32.
3. Blennow K, Brody D L, Kochanek P M, et al., Traumatic brain injuries. Nat Rev Dis Primers, 2016. 2: 16084.
4. Diaz-Arrastia R, Kochanek P M, Bergold P, et al., Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J Neurotrauma, 2014. 31(2): 135-58.
5. Chen G, Shi J, Hu Z, et al., Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm, 2008. 2008: 716458.
6. Siopi E, Llufriu-Daben G, Fanucchi F, et al., Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neurosci Lett, 2012. 511(2): 110-5.
7. Abdel Baki S G, Schwab B, Haber M, et al., Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS One, 2010. 5(8): e12490. 8. Sangobowale M A, Grin'kina N M, Whitney K, et al., Minocycline plus N-Acetylcysteine Reduce Behavioral Deficits and Improve Histology with a Clinically Useful Time Window. J Neurotrauma, 2018. 9. Kao Y C J L, C.F.; Chen, C.Y., Evolving Functional Connectivity in Rats following Mild Traumatic Brain Injury. ISMRM Preceeding, 2017.
10. Kao Y C J L, C.F.; Chen, C.Y., Low-frequency Fluctuations of Resting-state fMRI BOLD Signal after Experimental Mild Traumatic Brain Injury. ISMRM Preceeding, 2018.
11. Akiki T J, Averill C
L, Wrocklage K M, et al., Default mode network abnormalities in posttraumatic
stress disorder: A novel network-restricted topology approach. Neuroimage, 2018. 176: 489-498.
Figures