1924
Remote ischemic conditioning in a rat model of acute ischemic stroke: a two-centre study with translational longitudinal MRI
Marlene Wiart1, Maryna Basalay2, Fabien Chauveau3, Chloe Dumot1, Christelle Leon1, Camille Amaz4, Radu Bolbos5, Diana Cash6, Eugene Kim6, Tae-Hee Cho7, Norbert Nighoghossian7, Sean Davidson2, Michel Ovize1, and Derek Yellon2
1Université Lyon, CarMeN laboratory, Inserm U1060, Lyon, France, 2The Hatter Cardiovascular Institute, London, United Kingdom, 3Université Lyon, Lyon Neuroscience Research Center, CNRS UMR5292, Inserm U1028, Lyon, France, 4Clinical Investigation Center, HCL, Lyon, France, 5CERMEP-Imagerie du Vivant, Bron, France, 6Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom, 7Stroke Medicine, Université Lyon, CREATIS CNRS UMR 5220-INSERM U1206, Lyon, France
1Université Lyon, CarMeN laboratory, Inserm U1060, Lyon, France, 2The Hatter Cardiovascular Institute, London, United Kingdom, 3Université Lyon, Lyon Neuroscience Research Center, CNRS UMR5292, Inserm U1028, Lyon, France, 4Clinical Investigation Center, HCL, Lyon, France, 5CERMEP-Imagerie du Vivant, Bron, France, 6Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom, 7Stroke Medicine, Université Lyon, CREATIS CNRS UMR 5220-INSERM U1206, Lyon, France
Synopsis
The main objective of this study was to test the neuroprotective effects of remote ischemic conditioning (RIC: 4 cycles of 5-min hind limb ischemia interleaved with 5-min reperfusion) in a rat model of transient ischemic stroke (90 minutes) in a two-center study using translational MR imaging endpoints. Neuroscores and edema-corrected infarct size measured at 24h on T2-weighted MRI and expressed as percentage of the area at risk on per-occlusion MRI were significantly reduced in the RIC-treated group compared to the control group. The use of longitudinal MRI increases results robustness, which is greatly needed for successful RIC clinical translation.
Introduction
The main objective of this study was to test the neuroprotective effects of remote ischemic conditioning (RIC) in a rat model of acute ischaemic stroke in a two-center, international study, using translational MR imaging endpoints. RIC is a therapeutic approach whereby the application of brief episodes of ischemia/reperfusion to a tissue (e.g. the limb) can significantly protect a remote organ (e.g. the brain).1Methods
The study was approved by our local ethical committees, randomised and blinded. Minimum sample size was calculated a priori as 14 rats per groups. Eighty Sprague-Dawley male rats were used. Figure 1 shows the experimental design. In brief, MRI was first performed 45 minutes after middle cerebral artery occlusion (MCAO) by an intraluminal silicon-coated monofilament, using the following MRI protocol: MR angiography (MRA), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI). The primary purpose of per-occlusion MRI was: (i) to exclude animals with no cerebral ischemia (i.e., no abnormalities on PWI and DWI); and (ii) to quantify baseline DWI (apparent diffusion coefficient, ADC) and PWI lesion volumes. Reperfusion was obtained after 90 minutes of MCAO. RIC treatment was started 10 minutes before reperfusion and consisted of 4 cycles of 5-min left hind limb ischaemia interleaved with 5-min reperfusion,1 using an inflatable 12-mm cuff, which was inflated to 200 mmHg and subsequently deflated. A follow-up MRI was obtained for each animal at the end of the 24h recovery period, after being rated for neurofunctional outcome and before being euthanized. This second MRI primary aimed at evaluating the infarcted area and the extent of space-modifying edema in vivo.Data from all included animals in both centres were pooled for the final analysis. Safety data consisted in counting the number of animals that either died post-treatment or underwent hemorrhagic transformation (HT) in each group. The predetermined primary endpoint of the study was edema-corrected infarct size (IS) measured on T2WI at 24h and expressed as percentage of the area-at-risk (AAR) for each animal individually. The AAR was determined based on the hypoperfused region on PWI (the region that will get infarcted in the absence of reperfusion). The secondary endpoints were: (i) hemispheric space-modifying effect of edema (%HSE) estimated on MRI; (ii) infarct growth estimated on MRI, i.e. the difference between 24h T2WI lesion volume and per-occlusion DWI lesion volume; and (iii) neurofunctional outcome. In addition, we analysed lesion volumes on the photos of brain slices after TTC staining and compared the infarct volumes as evaluated by TTC and MRI. Following the Shapiro-Wilk test for normality of the data, the statistical analysis was performed by Wilcoxon-Mann-Whitney test.
Results
In total, 47 animals were analysed after applying all the exclusion criteria (N=23 in the control group and N=24 in the RIC group) (Figure 2). Two animals died in each treatment group and no animals underwent HT. Figure 3 shows typical examples of the longitudinal follow-up of one excluded animal and two included ones: one in the control group and one in the RIC group.Baseline data (ADC lesion and AAR) did not significantly differ between groups; however, there was a trend towards smaller ADC lesions in the RIC group (Figure 4A). Edema-corrected infarct size as % of AAR was significantly reduced in the RIC group (Figure 4A-B). RIC had no statistically significant effect on %HSE or infarct growth, although there was a trend in reduction for each parameter (Figure 4A). RIC significantly improved neuroscores (Figure 4A-C). There was an excellent correlation between infarct size measured by T2WI and that measured by TTC staining (0.87).
Because the difference in ADC lesion size at baseline may explain in part the difference in final infarct size, we performed a subgroup analysis by including only the most severe lesions, i.e. ADC lesions > 100 mm3. This stratification led to groups with fewer individuals (N=14 in the control group and N=10 in the RIC group) but with ADC lesions and AAR that were comparable between groups (Figure 5A). As for the whole cohort, the RIC effect was found significant again on both the primary endpoint and the neurofunctional secondary endpoint (Figure 5A-B-C).
Discussion
There are many benefits in performing per-occlusion MRI in pre-clinical neuroprotection studies: (i) the rigorous inclusion of animals; (ii) the comparison of baseline (pre-treatment) MRI data in order to make sure that ischemic areas were evenly distributed between groups; (iii) in-vivo edema correction and (iv) the use of translational imaging endpoints to measure outcome. The gold standard for measuring lesion sizes in pre-clinical studies of neuroprotection is TTC staining; we here confirm that MRI may be used as a surrogate marker. The advantage of using an MRI measurement of infarct size is two-fold: first, it better reflects what is measured in clinical trials, and second, the sampled brain may then be used for evaluating mechanistics hypothesis.Conclusion
RIC in the setting of acute stroke in rats is safe, reduces infarct size and improves functional recovery in a two-centre international study. The use of longitudinal MRI in pre-clinical international studies increases results robustness, which is greatly needed for successful clinical translation.Acknowledgements
This study was supported by the Hatter Foundation and the National Institute for Health Research Biomedical Research Centre (NIHR-BRC) (BRC233/CM/SD/101320) and performed within the framework of the RHU MARVELOUS (ANR-16-RHUS-0009) of University Claude Bernard Lyon 1 (UCBL), within the program “Investissements d'Avenir”.References
1. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: A novel therapy for acute stroke? Stroke. 2011;42:2960-2962Figures
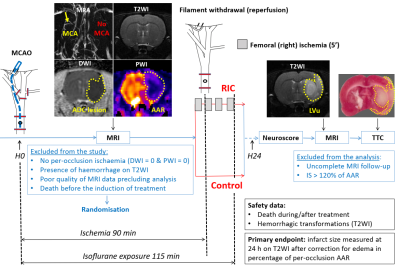
Figure 1- Study
design. tMCAO: transient middle cerebral artery
occlusion; PWI: perfusion-weighted MRI; MRA: magnetic resonance angiography;
DWI: diffusion-weighted MRI; ADC: apparent diffusion coefficient; T2WI:
T2-weighted MRI; RIC: remote ischemic conditioning; IS: infarct size; AAR: area
at risk; Lvu: lesion volume uncorrected.
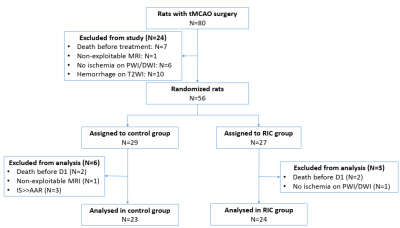
Figure 2- CONSORT-like
diagram of overall study. CONSORT
= Consolidated Standards of Reporting Trials; tMCAO: transient middle cerebral
artery occlusion; PWI: perfusion-weighted MRI; DWI: diffusion-weighted MRI;
T2WI: T2-weighted MRI; RIC: remote ischemic conditioning; IS: infarct size;
AAR: area at risk.
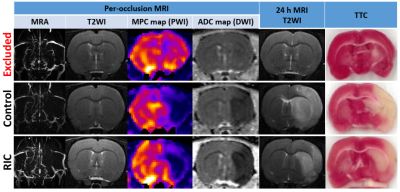
Figure 3- MRI
data of representative rats. Two
rats (2nd & 3rd row) presenting with the same MRI
features at admission. At 24h, the infarct had grown at the expense of penumbra in the control rat while
it remained within the ADC lesion borders in the animal treated with RIC. MRA:
magnetic resonance angiography; PWI: perfusion-weighted imaging; MPC: maximum
peak concentration; DWI: diffusion-weighted imaging; ADC: apparent diffusion
coefficient; T2WI: T2-weighted imaging; RIC: remote ischemic conditioning; AAR:
area at risk.
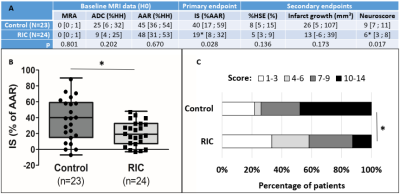
Figure 4- Baseline
data and efficacy outcome in overall propulation. (A) Median [interquartile range]. ADC: apparent
diffusion coefficient (hypointense lesion obtained from DWI data); AAR: area at
risk (hypoperfused region obtained from PWI data); %HH: percentage of healthy
hemisphere; IS: ischemic size; HSE: hemispheric space-modifying edema. (B)
Boxplots of MRI primary endpoint (infarct size); (C) Distribution of
neuroscores (higher score indicates more disability). *p<0.05
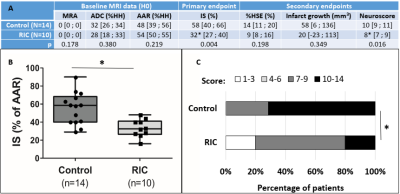
Figure 5- Baseline
data and efficacy outcome in the subgroup of rats with ADC lesion
> 100 mm3. (A) Median [interquartile range].
ADC: apparent diffusion coefficient (hypointense lesion obtained from DWI
data); AAR: area at risk (hypoperfused region obtained from PWI data); %HH:
percentage of healthy hemisphere; IS: ischemic size; HSE: hemispheric
space-modifying edema. (B) Boxplots of MRI primary endpoint (infarct size); (C)
Distribution of neuroscores (higher score indicates more disability). *p<0.05